A female patient presented to the clinic on a Tuesday morning after a holiday weekend with a sudden-onset red and painful right eye, reporting that she had also been exhibiting tearing and light sensitivity for the past day. She was a current contact lens wearer who had not visited the office since her last annual eye examination more than one year ago. Patient history included noncompliance with a monthly lens replacement schedule—the patient reported replacing her lenses every two months while also using numerous brands of multipurpose solution and failing to replace her contact lens case as recommended. The patient also acknowledged noncompliance with proper case cleaning and could not recall when she began using it; the case itself was covered with the dry and crusted remnants of her contact lens solution.
Following a thorough exam, the patient was diagnosed with microbial keratitis, empirically treated with a topical broad spectrum antibiotic eye drop, and given information on contact lens and lens case hygiene. She returned to the clinic for appropriate follow up care as recommended. Luckily for this patient, the location of her infection was peripheral and did not permanently affect her vision. After the infection resolved, she was re-fit into daily disposable contact lenses.
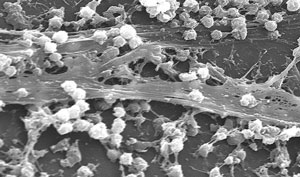 |
| Staphylococcus aureus biofilm on the surface of a medical device, a common concern in heath care generally, and among contact lens wearers in particular. |
Unfortunately, this presentation is all too commonly seen in contact lens patients who experience lens-related problems or infections. Though the majority of new contact lens patients are educated on the importance of proper care and replacement, in some cases they become complacent in their routine along the way, and these lapses enable infections to occur.
“Case” Report
Most patients are not aware of the danger inadequately managed contact lens cases in particular can present; without proper cleaning and storage procedures, free-floating microbes can easily attach themselves to the inside of a contact lens case and begin to secrete proteins that create a protective outer coating, known as a biofilm.1 This matrix-like structure can attach to both living and non-living surfaces, and has been found on other medical devices including prosthetic heart valves, coronary stents, prosthetic joints, cochlear and intraocular lens implants.1
The primary culprits of contact lens-associated microbial keratitis that can be found in contact lens case biofilms are Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Fusarium solani and Acanthamoeba. Together, these organisms account for more than 30,000 cases of microbial keratitis per year in the United States.1
For practitioners to adequately prevent or reduce biofilm formation, it is necessary to first understand how biofilms occur. Five stages of biofilm formation and transferability exist:
Stages 1 & 2. First, the biofilm begins as a reversible collection of floating cells in the solution near the case’s surface (stage 1), then as an attachment of the cells on the case (stage 2).
Stages 3 & 4. Next, more cell layers are constructed (stage 3), followed by the creation of a protective outer covering (stage 4).
Stage 5. Finally, the microbes detach and disperse into the solution within the case or onto the contact lens itself.1
A poorly fitting or potentially contaminated contact lens may alter the integrity of the corneal epithelium, allowing easy access from possible freeloading microbes from a biofilm to penetrate the compromised cornea and cause an infection.1 Furthermore, the presence of a contact lens on the eye can also negatively influence the antimicrobial action of the tear layer, causing an increase in the bacterial load and subsequent corneal invasion from transported biofilm microbes.1
 |
 |
 |
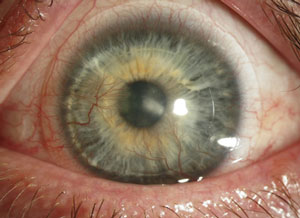
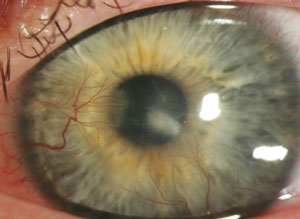
| A keratoconus patient with Type II diabetes who developed an infection following hybrid contact lens wear. Though most infections occur following soft contact lens wear, in rare cases improper care of GP and hybrid lenses can also lead to issues: this patient ran out of his prescribed hydrogen peroxide solution and elected to use a saline solution instead. Photos: Donna M. Wicker, OD |
Once a layer of cells has attached itself to the case and has begun to grow and reproduce, it is significantly more resistant to antimicrobials; as such, it may warrant further study. Current methods of attacking a biofilm—e.g., including bactericidal agents in contact lens solutions and cases, modifying contact lens case hygiene directions—are only designed to address the early stages of biofilm formation.
There’s the Rub
Both multipurpose and hydrogen peroxide-based contact lens solutions are designed to clean and disinfect contact lenses. For the most part, debris, makeup residue, proteins and mucus all need to be removed from the lens during the cleaning process—this must occur prior to the disinfection process in which the bacteria is destroyed.2 If the lenses are not thoroughly cleaned prior to disinfection, the antimicrobial action of the solution is reduced. This highlights the importance of mechanically cleaning, or rubbing, the contact lenses prior to soaking them in contact lens solutions for disinfection. Simply rubbing and rinsing lenses yields a 1-log reduction of microbes from the lens surface.
Common multipurpose solution disinfectants Polyquad (polyquaternium-1) and PHMB (polyhexamethylene biguanide) are found in different products, depending on the solution’s manufacturer, but the mechanism of action is the same: the chemical is incorporated into the cell membrane of the bacteria to cause membrane permeability and, ultimately, cell death.2 Hydrogen peroxide-based solutions, in contrast to multipurpose ones, use the oxidizing activity of the cleaning liquid to disrupt microbial DNA.
Contact lens solutions must meet the requirements of the International Standards Organization’s (ISO) standard 14729, “Microbial requirements and test methods for products and regimens for hygienic management of contact lenses.” These requirements address the antimicrobial efficacy against free-floating (planktonic) and non-attached cells of the following microbes: Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Fusarium solani and Candida albicans (Table 1).3 Recently completed research has demonstrated that current brands of multipurpose solutions are able to reduce biofilm formation.
Of course, past events like the Fusarium outbreak linked to ReNu with MoistureLoc (Bausch + Lomb) and the Acanthamoeba outbreak in connection with Complete MoisturePlus (Abbott) have taught practitioners that multipurpose solutions alone cannot be our only defense mechanism against biofilm formation. For example, ReNu with MoistureLoc was found to have adequate antimicrobial activity against free-floating Fusarium, but limited activity against a sessile (i.e., adherent) biofilm; as such, a Fusarium biofilm in a contact lens case with this solution was able to continue to grow, develop further antimicrobial resistance and subsequently cause an infection.1 Complete MoisturePlus was found to have limited activity against Acanthamoeba, allowing cysts to form, which led to keratitis.1 Both solutions were eventually voluntarily recalled from the market.
| Table 1. ISO 14729 Specific Test Microbes | |
| Bacteria | Pseudomonas aeruginosa Serratia marcescens Staphylococcus aureus |
| Fungi | Fusarium solani Candida albicans |
As more attention and interest is paid to alternative and complementary medicine and organic therapies, research is also being completed on the antimicrobial activity of certain types of plant oils in combination with contact lens solutions.4 For example, cinnamon oil has been found to contain cinnamic aldehyde and eugenol, which have a bactericidal effect on cell membranes and have been shown capable of detaching sessile bacteria from a surface.4 One study found that cinnamon oil combined with a multipurpose solution had an increased antimicrobial effect greater than that of cinnamon oil or the multipurpose solution alone. However, additional study is required to determine the risk of corneal toxicity, contact lens material damage or adverse reactions from mixing cinnamon oil in a contact lens solution.
On the Case
Most contact lens cases are comprised of a mix of three plastic polymers: polypropylene, styrene and polyethylene. During the production process, the contact lens solution manufacturer tests the efficacy of their solutions with a designated contact lens case; as such, it is important to remind patients to use the case included with the solution they purchase to ensure the cleaning and disinfecting properties of the solution are given the best possible environment to work within. In some instances, use of a specific solution with a contraindicated case can actually lessen its effectiveness.
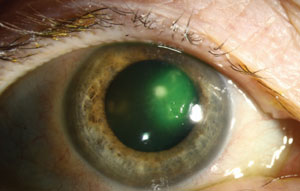 |
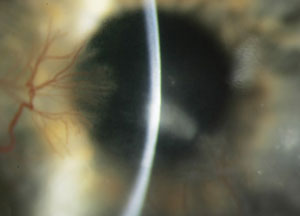
| Serratia marcescens corneal infection. Photo: Christine W. Sindt, OD |
Researchers have attempted to make special modifications to the material composition of the contact lens case to engender a bactericidal effect, namely by adding silver or organoselenium in an effort to reduce and prevent biofilm formation. Silver-lined cases work by slowly releasing silver ions into the lens solution during the disinfection process. Silver is known to have antimicrobial properties: it inhibits bacterial DNA replication and disrupts the cell membrane to cause cell death.5 Patients using silver-lined cases should soak their lenses for at least 24 hours, however, as only minimal antimicrobial effect was found at six- and 10-hour soaking times.5 Additionally, patient compliance with cleaning and replacing these cases is also an issue: over time, the silver ions are depleted from the case, limiting the antimicrobial activity.6
The addition of organoselenium into the polymer of the contact lens case was the subject of a recent study.7 The antimicrobial action of organoselenium serves as a catalyst to create superoxide free radicals that disrupt bacterial cell membranes. The advantage of the addition of organoselenium into the case composition over silver is that the organoselenium will not be depleted with time and use of the case, allowing for additional antimicrobial activity to occur despite the age of the case.
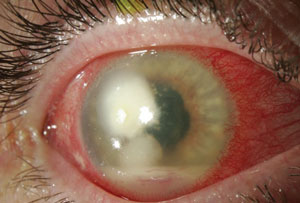 | 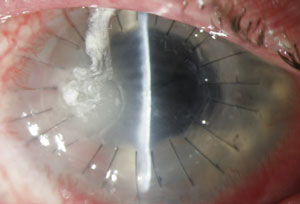 |
| Fusarium infection following contact lens wear. From left to right: (a) initial presentation and (b) post-corneal transplant treatment. Photos: Donna M. Wicker, OD | |
Back to the Future
Revisiting disinfection methods that have been used in the past may also be an effective means to counteract biofilm development. Twenty to 30 years ago, contact lenses were predominantly cleaned using a device that plugged into an electrical circuit to create heat. The advent and ease of use of multipurpose solutions caused the heat system to fall out of favor with many patients and providers; however, a recent study found that using a warming device that reached a temperature of 140˚F for three hours with a multipurpose solution was more effective against biofilm formation than the multipurpose solution alone.8 As such, further research is needed to develop a warming device that could be easily manufactured and potentially used by our contact lens patients.
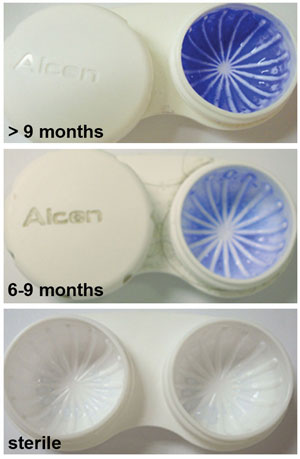 |
| Crystal violet staining of lens storage cases studied at a university campus showing correlation between age and staining severity. Staining was evident as early as six months. Those older than nine months showed dramatic increases in staining intensity.15 Photo: Danielle M. Robertson, OD, PhD |
The habits of noncompliant lens wearers have been thoroughly studied; hallmarks include reuse and topping off of solution, and not cleaning or replacing the case or contact lenses regularly. Some patients do some of these things, while others do all of them; regardless, not making these actions a habit can lead to a 4.4-fold higher risk of microbial keratitis.9 Interestingly, contact lens case contamination has been found in 58% to 85% of patients without any symptoms of infection.10 As such, proper contact lens case hygiene can have a significant impact on the reduction and removal of biofilm.
Practitioners should also refrain from recommending that patients discard used multipurpose solution from their lens cases, rinse the wells of the case with hot water or fresh multipurpose solution and let the case air dry, as exposure to water can place both the case and contact lenses within in contact with Acanthamoeba and other microbes. Research has indicated the most effective method for cleaning a contact lens case is to rub each well with a finger, rinse the case with fresh multipurpose solution, wipe the case with a clean tissue and then place it face-down to air dry.11,12 The Centers for Disease Control and the Food and Drug Administration have both updated their consumer safety websites with the new case cleaning recommendations.13,14
So what does this mean for practitioners and their patients? Unfortunately, there does not seem to be a quick fix for biofilm prevention or reduction. Research and development continues to be ongoing in an effort to create new solutions with improved antimicrobial efficacy, while other studies are addressing ways to add antimicrobial compounds into contact lens cases to limit cell adhesion. Still, continuing to teach new contact lens wearers and remind older ones about the importance of proper cleaning and replacement of not only their contact lenses but also their cases remains a priority.
Returning to the patient from the beginning, she reported that she had forgotten about cleaning the case, even though she admitted it looked rather dirty. She stated she would be compliant from now on for fear of another infection. Overall, however, it is hoped that it does not take an episode of infection for a patient to realize the importance of adequate care and replacement of contact lenses and cases.
1. Bispo PJM, Haas W, Gilmore MS. Biofilms in infections in the eye. Pathogens.2015;4:111-136.
2. Artini M, Cellini A, Scoarughi GL, Papa R, et al. Evaluation of contact lens multipurpose solutions on bacterial biofilm development. Eye Contact Lens. 2015;41:177-182.
3. Chang JML, McCanna DJ, Subbaraman LN, Jones LW. Efficacy of antimicrobial against biofilms of Achromobacter and Pseudomonas. Optom Vis Sci. 2015;92:506-513.
4. Bassyouni RH, Kamel Z, Abdelfatteh MM, Mostafa E. Cinnamon oil: a possible alternative for contact lens disinfection. Cont Lens Anterior Eye.2016;16:30001-7.
5. Dantam J, Zhu H, Stapleton F. Biocidal efficacy of silver-impregnated contact lens storage cases in vitro. Invest Ophthalmol Vis Sci.2011;52:51-57.
6. Wu YT, Zhu H, Willcox M, Stapleton F. Impact of cleaning regimens in silver-impregnated and hydrogen peroxide cases. Eye Contact Lens. 2011;37:365-369.
7. Tran PL, Huynh E, Pham P, Lacky B, et al. Organoselenium polymer inhibits biofilm formation in the polypropylene contact lens case material. Eye Contact Lens.2016;1:1-6.
8. Willcox MDP, Zhu H, Vijay AK. Effect of a warming device on contact lens case contamination. Eye Contact Lens. 2012;38:394-399.
9. Uno T, Ohashi Y, Imayasu M. Antimicrobial efficacy tests of multipurpose contact lens case solutions simulating poor contact lens hygiene behaviors. Eye Contact Lens. 2012;38:388-393.
10. Vijay AK, Wilcox M, Zhu H, Stapleton F. Contact lens storage case hygiene practice and storage case contamination. Eye Contact Lens. 2015;41:91-7.
11. Wu YT, Zhu H, Wilcox M, Stapleton F. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci. 2010;51:6329-6333.
12. Wu YT, Zhu H, Wilcox M, Stapleton F. Impact of air-drying lens cases in various locations and positions. Optom Vis Sci. 2010;87:465-468.
13. Centers for Disease Control and Prevention. Contact lens case systems and solutions. Available at www.cdc.gov/contactlenses/care-systems.html. Accessed April 25, 2016.
14. U.S. Food and Drug Administration. Focusing on contact lens safety. Available at www.fda.gov/ForConsumers/ConsumerUpdates/ucm048893.htm. Accessed April 25, 2016.
15. Burnham GW, Cavanagh HD, Robertson DM. The impact of cellular debris on Pseudomonas aeruginosa adherence to silicone hydrogel contact lenses and contact lens storage cases. Eye Contact Lens 2012;38:7-15.


