Microbial keratitis affects an estimated 30,000 Americans annually.1 Typically, the event occurs in eyes susceptible to infection by some pre-existing condition and only rarely occurs in an otherwise unaffected eye, due to the cornea’s robust defenses against infection.1,2 Any condition that causes, or leads to epithelial corneal damage, in which laceration of the basal laminar layer occurs, increases one’s risk for developing bacterial corneal ulcers, also known as bacterial or ulcerative keratitis.
In general, corneal infections typically arise from the same species of bacteria normally found on the lids, periocular skin, conjunctival sac or in adjacent nasal passages. The most common organisms found in the uncompromised, healthy cornea include Staphylococcus, Streptococcus, Pseudomonas, Moraxella and enterobacteriaceae such as Serratia marcescens and Klebsiella pneumonia. Proteus may also be encountered in a compromised cornea.
Gram-positive Staphylococcus aureus is the most common organism identified in North America, while gram-negative Pseudomonas aeruginosa is the most common underlying etiology documented in contact lens-associated corneal ulcers. Streptococcus pneumoniae is more prevalent in the developing world.1,3-4
Other, less common causes of microbial contact lens-related keratitis include infections resulting from the presence of fungi (i.e., Fusarium, Aspergillus, Candida) and protozoa (i.e., Acanthamoeba, microsporidia, rhinosporidia).4
 | |
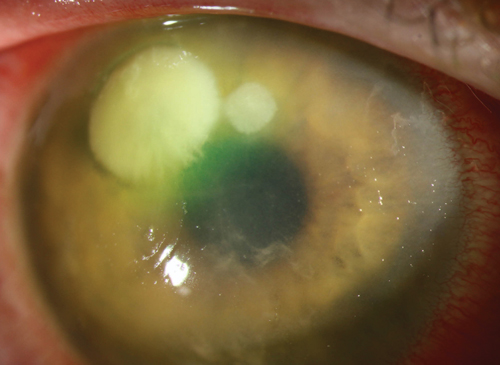
| Fig. 1. A Staphylococcus ulcer is typically round, with well-demarcated discrete borders. Photo: Christine W. Sindt, OD |
Clues in the Presentation
Signs and symptoms of bacterial keratitis typically commence within 24 hours of infection. Common symptoms include photophobia, decreased visual acuity, redness, discharge, eyelid swelling, and pain of varying degrees. Patients with a Moraxella-associated ulcer or with corneal hypoesthesia may not report significant pain.2,4
A significant number of bacterial keratitis cases result in loss of vision due to corneal scarring and irregularities.1 Bacterial ulcers can occur at any location on the cornea, but those that are central or paracentral pose the greatest risk to vision even if the infecting organism is successfully treated.1
Typically, patients develop moderate to severe lid and conjunctival edema and inflammation, along with a mucopurulent discharge. Often, the stroma exhibits a dense, gray-white infiltrate with indistinct margins, surrounding corneal edema and an overlying, ulcerated, epithelial defect that stains with fluorescein. There may be an associated mild to severe anterior chamber reaction, which could lead to hypopyon formation, elevated IOP, cataract and synechiae (Table 1). Other potential findings include descemetoceles and corneal perforation, with the potential for endophthalmitis to develop and result in loss of the eye.1-4
| Table 1. Differentiating Sterile and Infectious Infiltrates | |||||||
| Location | Number of Lesions | Lesion Size | Degree of Staining | Pain Level | Injection | A/C Reaction | |
| Infectious | Typically central 6mm | Typically single | Greater than 1mm | Staining Equal to Lesion Size | Severe | Diffuse, Severe | +Cells and Flare |
| Sterile | Peripheral; located at 2, 4, 8, and 10 o'clock; clear "lucid" zone between cornea and limbus | Single or Multiple | 0.1mm to 1.5mm | Staining Less than Lesion | Mild to Moderate | Localized, Mild to Moderate | No/Minimal Cell and Flare |
Clinically, there are some differences in presentation that can aid in determining the causative agent and, subsequently, the best initial treatment. Note, however, a laboratory culture is needed to make a definitive diagnosis. A corneal ulcer caused by Gram-positive organisms, such as Staphylococcus, will present with a yellow and gray-white area of infiltration located directly beneath an epithelial defect. Generally, these lesions are well-demarcated, discrete round or oval-shaped “dry” abrasions that may be associated with a severe anterior chamber reaction (Figure 1).
Ulcers caused by Gram-negative bacteria, in contrast, are more diffuse in presentation with a “soupy” or “wet” appearance. The mucopurulent discharge seen in P. aeruginosa, for example, is yellow-green and can cling to the ulcer’s surface. Similarly gray-white in color, they initially involve the central cornea, but then progress rapidly outwards to encompass the entire cornea. Quite often, the anterior chamber reaction produced by gram-negative bacteria is intense, with hypopyon formation (Figure 2).2-3
Investigating Infiltrates
When evaluating a patient with corneal infiltrates, determine whether it is sterile or the result of an infectious process, as the clinical course and prognosis can be quite different. Sterile corneal infiltrates develop from one of a number of insults associated with contact lens wear, including trapped debris, chemical toxicity, hypoxia and hypersensitivity. They may also be the result of an immune reaction to exotoxins from staphylococci colonizing the sebaceous gland openings of the eyelid margin, known as staphylococcal marginal keratitis. A variety of systemic diseases (e.g., rheuma toid arthritis, polyarteritis nodosa and sarcoidosis) may also lead to stromal infiltration. Sterile infiltrates appear as white lesions and represent inflammatory cells within the collagen matrix of the corneal anterior stroma.1-2,4
Patients are usually asymptomatic, although some may present with a small degree of pain, discomfort, tearing or photophobia (Table 2). A careful history regarding contact lens hygiene should be obtained. Successful treatment can be achieved with the initiation of topical corticosteroids and even the concomitant use of topical antibiotics to help control ocular surface and eyelid bacterial flora. It is also recommended that patients refrain from contact lens wear for a short period and brush up on proper eyelid and contact lens hygiene.2,4
| Risk of Vision Loss | Low | Moderate | Severe and Sight-Threatening |
| Clinical Features | Small, non-staining peripheral infiltrate; minimal A/C reaction; (-)discharge. | Peripheral infiltrate 1mm to 1.5mm in size, or smaller infiltrate with associated epithelial defect. | Greater than 1mm to 2mm in size; central; unresponsive to initial therapy. |
| Antibiotic(s) to Use | Monotherapy with topical FQL (i.e., moxifloxacin, gatifloxacin, besifloxacin) | Monotherapy with topical FQL. | Combination fortified aminoglycoside-cephalosporin agents. |
| Dosing Schedule | One drop every 1-2 hours. | One drop every hour around the clock with loading dose of five drops separated by five minutes each. | Fortified tobramycin or gentamicin (15mg/mL) alternated with fortified cefazolin (50 mg/mL) every 30 minutes. |
| Other Considerations | For contact lens wearers, consider antibiotic ointment at nighttime (i.e., tobramycin, bacitracin/polymyxin B). | Consider adding an antibiotic ointment QHS or BID. | Vancomycin can be used instead of cefazolin in resistant or unresponsive cases, or when the patient is allergic to penicillin or cephalosporins. Also, consider antibiotic ointment QHS or BID. |
Culturing
Identifying the offending organism in bacterial keratitis is important when determining the appropriate treatment regimen and preventing potentially severe complications. Culturing is the only means of determining sensitivity to antibiotics, which allows for the selection of the most appropriate antibiotic and prevents overuse of ineffective antibiotics.1,3 Having said that, the majority of community-acquired cases of bacterial keratitis are managed without smears or cultures and resolve with empiric therapy.1,4 Some indications for when culture and sensitivity and Gram stain testing should be obtained include: cases that involve a large, central corneal infiltrate that extends to the middle to deep stroma; immunocompromised or hospitalized patients; a history of organic trauma, those that are chronic in nature or unresponsive to broad-spectrum antibiotic therapy; sight-threatening lesions; sclera extension is present or cases that have any atypical clinical features suggestive of another etiology.1
Culture specimens should be obtained from the conjunctiva and lid margins of infected eyes, as well as from the leading edge and base of the corneal ulcer using a calcium alginate swab moistened with thioglycolate or trypticase soy broth. A new swab should be used for each area cultured. The use of anesthetics is not recommended for conjunctiva or lid margins, as the preservatives may cause a decreased yield of live organisms; however, an agent such as proparacaine hydrochloride 0.5% should be used in obtaining corneal specimens for patient comfort. Cultures can also be obtained using a sterile transport swab in a mini-culture tube (Figure 3). Specimens can then be plated onto culture media and also sterile glass slides for Gram and Giemsa staining. Typically, blood agar is inoculated first, as it readily supports the growth of most corneal pathogens. Other media available include chocolate agar, Sabourand’s agar, thioglycolate broth, and brain-heart infusion broth. Information from cultures is usually available within 24 to 48 hours.1-3 Plates looking for fungal growth should be held for two weeks or longer.
Treatment
Initial therapy for the treatment of bacterial keratitis depends on severity and clinical presentation. Studies comparing the efficacy of fluoroquinolones (FQs) with fortified antibiotics have found that empiric treatment with FQs seems to be at least as effective as combined fortified antibiotics in managing bacterial keratitis.6,8 FQs offer the advantage of good ocular penetration, broad-spectrum activity, less risk of ocular discomfort/toxicity, lower dispensing cost and ready availability.6-8 For these reasons, they have become the most widely used options in the treatment of bacterial keratitis, although the newest FQs are not FDA-approved for this purpose.
Further research has delineated that the fourth-generation fluoroquinolones, moxifloxacin and gatifloxacin, have better coverage of Gram-positive organisms, while the older second-generation FQ, ciprofloxacin, works better for Gram-negative organisms, including Pseudomonas.2,9-10 Besifloxacin 0.6%, a chlorofluoroquinolone, is the newest topical antimicrobial available. The gel-forming polymer (i.e., Durasite, InSite Vision) used in its preparation yields greater ocular surface retention time compared with others in its class.
 | |
| Fig. 2. Gram-negative bacteria can induce an intense anterior chamber reaction, as in this Pseudomonas ulcer. Photo: Christine W. Sindt, OD |
Although currently only approved to treat bacterial conjunctivitis, besifloxacin has been postulated in rabbit models to have some treatment efficacy in bacterial keratitis even in the presence of epithelial disruption.7 In addition, this drug has shown greater in vitro potency compared to other FQs, particularly among MRSA (methicillin-resistant S. aureus) and MRSE (methicillin-resistant S. epidermidis) species, although further investigation is needed.1,7,11-12 There are some concerns of increased risk of corneal perforation and delay in epithelial closure with fluoroquinolone use, as they may alter corneal collagen or keratocyte function, however, this has not yet been confirmed via a randomized controlled trial.1,13-15
For suspected or confirmed cases of Pseudomonas, gatifloxacin and ciprofloxacin provide better coverage than other FQs.1,4,10-11 The use of a fluoroquinolone in conjunction with tobramycin or bacitracin/polymyxin B provides an even broader spectrum of coverage and further decreases the development of resistant bacteria. Cycloplegia agents may be used to decrease synechia formation and pain, and are indicated when anterior chamber inflammation is present. Topical scopolamine 0.25%, homatropine 5% or atropine 1% can be used two to three times daily.1-3,17
An initial loading dose of antibiotic is critical for any moderate to severe corneal ulcers. Following this, patients should be monitored daily until a positive response to treatment is observed, such as re-epithelialization, reduced pain, smaller ulcer/infiltrate size and depth and/or an improvement in iritis. The IOP may be elevated if there is a secondary iritis, and if so, must be treated. Avoid prostaglandins and miotics, as they can increase inflammation. If a positive response is appreciated, the antibiotic regimen can be tapered, but should not be set below the minimum inhibitory concentration (MIC) for the chosen antibiotic—usually a TID and QID dosing—to decrease the chance of developing resistance. Consider hospitalization in severe cases or if there are concerns of noncompliance.2-3,10,18 If the lesion does not show some improvement in four to seven days, consider other, less common causes of keratitis.19
Although somewhat controversial, adjunctive therapy with topical corticosteroids can be beneficial in the treatment of some bacterial keratitis (avoid steroids if the infectious agent is Mycobacterium or Nocardia). Subsequent trials and analyses of the Steroids for Corneal Ulcers Trial (SCUT) support the practice of initiating topical steroids early in the course of the disease. After approximately 24 to 48 hours of appropriate antibiotic therapy, a topical corticosteroid (prednisolone acetate 1%, difluprednate 0.05%, or loteprednol etabonate 0.5%) can be started BID to QID to help speed resolution and hopefully reduce corneal scarring.2,17,20-21
Novel Therapies
Given the increasing prevalence of MRSA, as well as resistance to cephalosporins and fluoroquinolones, studies are emerging to find novel targets for antimicrobial therapy. Resistance occurs when there is a single- or multi-step mutation in the genes encoding the target enzymes.18 The older fluoroquinolones (i.e., ciprofloxacin and ofloxacin) preferentially inhibit topoisomerase IV of gram-positive bacteria, whereas the newer FQs (i.e., moxifloxacin and gatifloxacin) exhibit a more balanced inhibition of both DNA gyrase and topoisomerase IV. This requires a double step mutation to result in resistance. Agents such as besofloxacin, with only a topical formulation available, are less susceptible to resistance compared with antibiotics with both topical and oral formulations.1,10-11,18,22 Finally, some older, less frequently used drugs such as bacitracin, gentamycin and sulfacetamide may prove successful if resistance is encountered. Topical, fortified vancomycin is the last resort drop for MRSA or any gram-positive resistant bacteria. As noted above, culture and sensitivity should be used to tailor the appropriate therapy.19
Corneal crosslinking uses ultraviolet-A radiation and riboflavin to produce covalent bonds, or “cross-links,” in the corneal stroma by way of photochemical reactions. In vitro experiments have shown that stiffening the corneal stroma in effect stabilizes it and increases its resistance to enzymatic bacteria degradation, avoiding progression to corneal melting. Currently, the indication for this treatment is set aside for those cases that are recurrent or unresponsive to traditional antimicrobial therapy.10,23
Many studies reveal success in using amniotic membrane transplantation (AMT) in the treatment of infectious keratitis and corneal perforation.24 The amniotic membrane allows for epithelial cell migration, promotes re-epithelialization, inhibits inflammation and may have an antimicrobial effect. Thus, AMT could be a viable option if medical treatment fails or there is corneal perforation.24
Other adjunctive therapies can be used to support corneal surface healing once the lesion is sterile. If healing is impaired, consider bandage contact lenses, autologous serum eye drops, punctal plugs or tarsorrhaphy. Oral therapy with doxycycline or vitamin C is helpful in cases of stromal melt. Following healing, if the patient is left with an irregular, scarred cornea, a GP or scleral lens may be indicated to provide the best vision. Other options for managing a scarred cornea include phototherapeutic keratectomy or corneal transplant.16,19
Bacterial keratitis can be visually devastating if not identified early and treated immediately. In many cases, empiric treatment with fourth-generation fluoroquinolones can be successful, especially when combined with an antibiotic ointment of a different class. Practitioners must always be prudent in determining the causative organism if patients do respond as expected to initial therapy, and should consider culturing in all cases.
Dr. Murphy practices at the White River Junction VAMC in Vermont, where she serves as an attending optometrist for student interns and residents. She is also the associate director of the clinic's low vision and blind rehabilitation services.
Dr. Frick practices at the White River Junction, VT VAMC. He is an award-winning educator who coordinates the student intern program and also teaches residents in the VA hospital. He travels to Latin America annually for SVOSH trips.
1. Garrett S. Bacterial Keratitis. Preferred Practice Pattern, American Academy of Ophthalmology, 2013.
2. Bartlett J, Jaanus S. Diseases of the Cornea. Clinical Ocular Pharmacology, 5th Edition. Chapter 20, pgs 519-525. Butterworth-Heinemann, St. Louis, MO, 2008.
3. Abbott R, Halfpenny C, Zegans M, Kremer P. acterial Corneal Ulcers. Duane’s Clinical Ophthalmology, 12th edition. Volume 4, Chapter 18. Lippincott, Williams & Wilkins, Philadelphia, PA, 2013.
4. DeLoss K, Soong H, Hood C. Complications of contact lenses. UpToDate, November 4, 2014. www.uptodate.com.
5. Collier, SA, Gronostaj MP, MacGurn AK, et al. Estimated Burden of Keratitis – United States, 2010. Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention. Nov 2014:63(45);1027-1030.
6. McDonald E, Ram F, Patel D, McGhee C. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomized controlled trials. Br J Ophthalmol 2014;98:1740-1477.
7. Chung JL, Lim EH, Song SW, et al. Comparative Intraocular Penetration of 4 Fluoroquinolones After Topical Instillation. Cornea, July 2013;32(7): 1046-1051.
8. Hanet MS, Jamart J, Chaves AP. Fluoroquinolones or fortified antibiotics for treating bacterial keratitis: systematic review and meta-analysis of comparative studies. Can J Ophthalmol, Dec 2012;47(6):493-499.
9. Gerstenblith A, Rabinowitz M. The Will’s Eye Manual – Office & Emergency Room Diagnosis and Treatment of Eye Disease, 6th edition. Lippincott Williams & Wilkins, Philadelphia, PA, 2012.
10. Wong, RLM, Gangwani RA, Yu L, Lai J. New Treatments for Bacterial Keratitis: Review Article. Journal of Ophthalmology, Volume 2012.
11. O’Brien T. Besifloxacin ophthalmic suspension, 0.5%: a novel topical fluoroquinolone for bacterial conjunctivitis. Adv The, 2012;29(6):473-490.
12. Schechter BA, Parekh JG, Trattler W. Besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial keratitis: a retrospective safety surveillance study. J Ocul Pharmacol Ther. 2015 Mar;31(2):114-21.
13. Gangopadhyay N, Daniell M, Weih L, Taylor HR. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. Br J Ophthalmol 2000;84:378-384.
14. Mallari PL, McCarty DJ, Daniell M, Taylor H. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol. 2001 Jan;131(1):131-3.
15. Talamo, J, Hatch K, Woodcock E. Delayed epithelial closure after PRK associated with topical besifloxacin use. Cornea. October 2013;32(10): 1365-1368.
16. Melton R, Thomas R. Eye Care Antibiotics: Cinical Guide to Ophthalmic Drugs. A Supplement to Review of Optometry. May 2015, 24-30.
17. Sowka JW, Gurwood AS, Kabat AG. Bacterial Keratitis. The Handbook of Ocular Disease Management: 17th Edition. Review of Optometry. June 2015;35A-37A.
18. Agarwal T, et al. Moxifloxacin resistance: intrinsic to antibiotic or related to mutation? Optometry and Vision Science. Dec 2012;89(12): 1721-1724.
19. Weiner, Gabrielle. Confronting Corneal Ulcers – Pinpointing Etiology is Crucial for Treatment Decision Making. EyeNet Magazine. July 2012.
20. Srinivasan M, Mascarenhas J, Rajaraman R, et al. The Steroids for corneal ulcers trial (SCUT): secondary 12-month clinical outcomes of a randomized controlled trial. Am J Ophthalmol. 2014;157(2):327-33.
21. Ray KJ, Srinivasan M, Mascarenhas J, et al. Early addition of topical corticosteroids in the treatment of bacterial keratitis. JAMA Ophthalmol. 2014 June;132(6):737-41.
22. Suzuki T, MD, PhD. A New Target for Staphylococcus aureus Associated With Keratitis. Cornea, Oct 2011;30(10): suppl.1: S34-S40.
23. Alio J, Abbouda A, Valle D, Castillo J, and Fernandez J. Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. Journal of Ophthalmic Inflammation and Infection 2013: 3(47): 1-7.
24. Abdulhalim B-EH, Wagih M, Gad A, Boghdadi G, Nagy R. Amniotic membrane graft to conjunctival flap in treatment of non-viral resistant infectious keratitis: a randomized clinical study. Br J Ophthalmol 2015;99:59-63.


