Invented in 1911 by Swedish ophthalmologist Allvar Gullstrand, the first slit lamp biomicroscope was a bright rectangular beam of light used to examine the cornea and crystalline lens.1 While the slit lamp was a cutting-edge device at the time, technology for corneal analysis has since evolved tremendously. Not only are the newest devices capable of examining the cornea at its cellular level, they also use sophisticated software to detect the earliest signs of corneal issues. As with all technology, however, each device has its advantages and limitations. This article will discuss the latest diagnostic imaging technology for corneal analysis, including corneal topography, corneal tomography, Scheimpflug-based noncontact tonometry, anterior segment optical coherence tomography, ultrasound biomicroscopy and confocal microscopy.
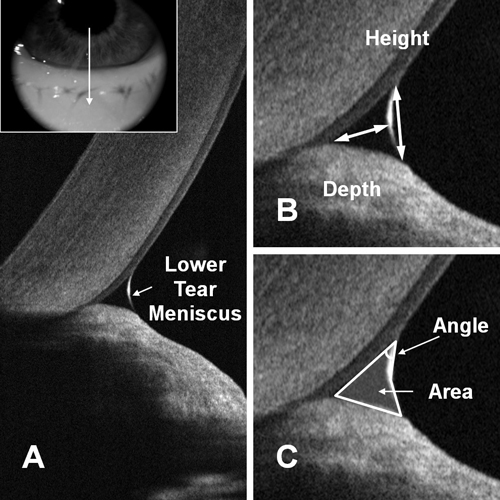 | |
| Measuring the tear meniscus with OCT, a recent innovation that allows for noninvasive analysis of the tear film characteristics. Photo: Yan Li, PhD, and David Huang, MD, PhD, Casey Eye Institute, Portland, OR. |
Corneal Topography
This mainstay of most eye care practices analyzes the anterior surface of the eye by reflecting a series of concentric circles, known as Placido rings, off of the cornea. A digital camera captures the reflected pattern and any deviations from the original image are processed by software to calculate the shape of the cornea. Placido-based corneal topography is considered advantageous for its ability to quickly capture a clear image without contact; however, its scope is limited to the anterior cornea and its premise is based upon the assumption that the cornea is prolate. Non-prolate corneas or irregular corneal surfaces are often misdiagnosed or broadly categorized as “irregular” with no further data given.2 Additionally, the patient must have an intact epithelial surface and tear film for the instrument to obtain a clear image. Poor tear films disrupt the smooth surface of the cornea and reduce corneal topography’s image quality.
Interestingly, the necessity of an intact tear film has led to the use of corneal topography in dry eye evaluations.3-5 Because tear film stability is needed to produce clear images, any irregularity caused by a poor tear film is captured in a series of corneal topography images over a period of 10 seconds. The images are then analyzed and quantified with a tear stability analysis software program to objectively measure the stability of the tear film on the eye.
While many corneal topographers exist, one of the newest devices available on the market, a color LED corneal topographer, is considered particularly novel. Similar to the VU (Vrije Universiteit) topographer, which projects a color-coded checkerboard on the cornea instead of Placido rings, the Cassini (i-Optics) shines up to 700 red, yellow and green spots from hundreds of LEDs on up to 8.5mm of the corneal diameter.6,7 Reflections of the spots are captured from seven different angles with a number of cameras, and ray tracing is done for every three spots, allowing for multiple elevations to be defined across the cornea without the assumption that the cornea is prolate. This allows for higher predictability in central irregular corneas as compared to Placido-based topographers.8 Its reliability in measuring keratometry on normal corneas was repeatable and comparable to the Pentacam (Oculus), though it provided higher keratometry values than both the Pentacam and EyeSys (EyeSys Vision).9 In irregular corneas, it showed high specificity in estimating corneal keratometry as compared to the manual keratometer and the Pentacam, Orbscan II (Bausch + Lomb), Galilei (Ziemer Ophthalmic Systems) and Sirius (Schwind) corneal topographers.10
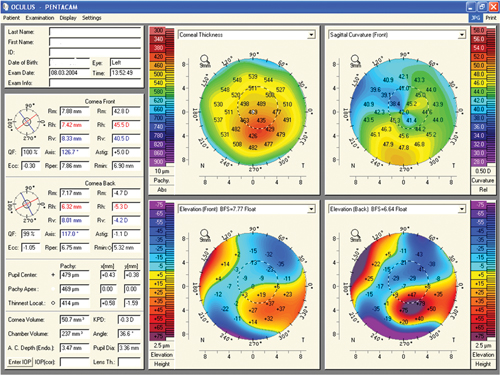 | |
| Pentacam readout of a keratoconus patient. Photo: Gregory W. DeNaeyer, OD, and S. Barry Eiden, OD |
Corneal Tomography
Whereas corneal topographers are limited to evaluating the anterior surface of the cornea, corneal tomographers can measure the elevation maps of both the anterior and posterior cornea to create three-dimensional images. Corneal tomographers can be used to evaluate corneal thickness, anterior chamber depth, lens thickness and lens opacification, as well as create cross-sectional images of the cornea and topographies of the anterior surface of the lens and iris. Because corneal tomographers can easily detect the higher and lower aberrations of irregular corneas, they are now being used to guide combination collagen crosslinking and photorefractive keratectomy surgical procedures in keratoconic eyes for improved visual outcomes.11
Three types of corneal tomographers exist: those that use slit scanning (i.e., Orbscan II), those with Scheimpflug imaging (i.e., Pentacam) and those that combine Scheimpflug cameras and Placido topography (i.e., Galilei, Sirius and TMS-5/Tomey). All are limited, however, by longer procedure times, which necessitates longer steady eye fixation as compared to Placido-based corneal topographers.
• Slit Scanning. Similar to a slit lamp’s light slits, optical slits are projected along multiple points across the cornea at a fixed 45 degree angle. The reflections of the slits of light are captured by a digital video camera and analyzed to reconstruct the anterior and posterior cornea. However, these tomographers are limited by their accuracy in measuring corneal thickness in irregular corneas and those with corneal opacities.12
• Scheimpflug Imaging. A rotating Scheimpflug camera captures 25 to 50 cross-sectional slits in a two-second scan across the corneal surface. A second stationary camera centered at the pupil aligns the images and monitors ocular fixation. Both sets of images are then analyzed to reconstruct the corneal topographies and produce a high-resolution three-dimensional image of the anterior segment, from the anterior cornea to the posterior crystalline lens. Some tomographers equipped with Scheimpflug imaging (e.g., Pentacam) also provide corneal wavefront analysis, densitometry and information about the anterior chamber.
• Scheimpflug and Placido Imaging. Tomographers with this combination incorporate two Scheimpflug rotating cameras and Placido disc imaging to collect 122,000 data points on the posterior cornea, anterior chamber, iris, pupil and lens. The scan takes approximately one to two seconds and records a 14mm diameter of the cornea.
• Scheimpflug-based Noncontact Tonometry. The biomechanics of the cornea can be better understood by measuring its deformation during noncontact applanation, such as during air puff tonometry. The measurement of corneal deformation induced by refractive surgery procedures may help predict the development of ectasias. The first device to record this effect, the Ocular Response Analyzer (Reichert), measures the reflected infrared light from the deformed corneal surface. Studies using the ORA have shown statistically different biomechanical strengths between normal and keratoconic eyes and post-LASIK.13,14
The new Corvis ST (Oculus) noncontact tonometer integrates a high-speed Scheimpflug camera that takes approximately 4,300 frames per second to measure the corneal deformation. Thinner and hence less rigid corneas would have a longer applanation time. The Corvis ST tonometer’s corneal thickness measurements were similar to those measured with ultrasound pachymetry.15
Optical Coherence Tomography (OCT)
Similar to an ultrasound—albeit reflecting light instead of sound—anterior segment OCT noninvasively captures high-resolution cross-sections of the eye by reflecting near-infrared light waves, which are scattered on the ocular structures. Low coherence interferometry analyzes the delay and intensity of the reflected light beams and compares it to light that has traveled along a referenced path. The axial scans (A-scans) are then combined to create a three-dimensional image, allowing for cross-sectional views of the cornea and anterior chamber.
These cellular level images provide more in-depth evaluations of the cornea, anterior chamber and corneal thickness, allowing for the study of tear film dynamics and the diagnosis of corneal abnormalities. OCTs are as efficient as ultrasound biomicroscopy for measuring pachymetry with the advantage of being noncontact, allowing them to be used to view ocular structures in their natural state.16 Additionally, because corneal opacities reflect and scatter the reflected light beams, OCTs are also useful in measuring opacity depths and sizes.
Several categories of OCT instruments exist. The most common distinction is time-domain vs. spectral-domain (also called Fourier-domain).
• Time-Domain (TD). TD-OCTs are older generation tomographers that create serial A-scans as a mechanical arm with a reference mirror moves across the cornea. These devices are particularly sensitive to motion errors, so the A-scans must be well aligned to produce clear images. Additionally, due to the mechanical limitation of the reference mirror, there is a lag in scanning time of 2,000 axial scans per second. However, while TD-OCTs are slower than spectral-domain OCTs, their lower wavelength can penetrate deeper into the eye—as much as 18µm through tissues such as the sclera, iris and opacified corneas—making them valuable instruments for anterior segment care. Examples include Visante and Stratus (both from Carl Zeiss Meditec).
• Spectral-Domain (SD)/Fourier-Domain (FD). SD-OCTs are newer tomographers capable of producing higher-resolution images than their TD-OCT predecessors. Instead of the mechanical sweeping arm of the TD-OCT, reflections from a reference light generate spectral interference fringes that are captured through an FD spectrometer and a high-resolution charge-coupled device camera. The signals are captured in parallel rather than serially and calculated electronically instead of mechanically, allowing for faster imaging speeds (up to 26,000 axial scans per second). The speed of image acquisition is somewhat limited to the camera’s frame capture rate and the computer speed of the Fourier calculation; however, the high imaging speed combined with the SD-OCT’s ability to track eye movement minimizes motion artifacts and hence improves repeatability of results.
Both SD-OCT and TD-OCT instruments can be used for similar evaluations of a high-resolution image of the cornea and other anterior segment structures, as well as recording pachymetry.17,18 The higher resolution imaging capabilities of SD-OCTs, however, improves sensitivity and repeatability of measured results. A custom-built, ultrahigh-resolution SD-OCT can evaluate epithelial, Bowman’s layer or endothelial conditions up to 2µm.19 OCTs can also measure corneal pachymetry over contact lenses, allowing for the creation of corneal thickness measurements caused by contact lens wear.20 Studies have found that SD-OCT has good correlation to confocal microscopy when evaluating the cornea demarcation line during corneal collagen crosslinking.21 SD-OCTs are also used to assess the location of Descemet’s membrane to guide manual dissection during deep anterior lamellar keratoplasty.22
The primary limitation of SD-OCT is the need for clear corneas to achieve accurate imaging. One study found that SD-OCT could not image corneas obscured by vascularized, densely inflamed or thick lesions such as pterygia. Similarly, underlying sclera could not be imaged in detail beneath conjunctival lesions.23
Newer platforms being developed—largely for posterior segment imaging—use concepts such as swept-source (i.e., multi-frequency) OCT or enhanced depth imaging (a modification of conventional SD-OCT) to capture the choroid and improve image resolution.
Ultrasound Biomicroscopy (UBM)
UBM noninvasively examines the anterior cornea through the use of ultra-high frequency acoustic waves (as opposed to light waves in OCT). An eyecup filled with a coupling medium (generally saline or methylcellulose) is applied on an anesthetized cornea when a patient is in a supine position. The transducer immersed in the coupling medium emits the sound waves. Each A-scan is mapped and the real-time image is displayed on a video monitor.
Recent advances in UBM have contributed to its rise in popularity amongst clinicians, as higher quality images are being produced, correlating to diagnoses that previous imaging technologies could not assess. UBM may offer better imaging quality of corneal wounds than traditional technologies. A study evaluating the results of a standard slit lamp exam compared to an UBM examination on a full-thickness corneal laceration found that while a slit lamp examination could not accurately discern the architecture of the corneal laceration due to the severe irregularity and localized corneal edema, the UBM examination defined the edges of the wound and identified the overlapping cornea.24 UBM also has better imaging quality posterior to the iris pigment epithelium compared to AS-OCT.
UBM allows for an in-depth assessment of anatomical and pathophysiological changes of the cornea, iris, sclera, ciliary body and zonules. Because the acoustic waves only penetrate 4mm to 5mm, however, visualization of the eye is restricted to the anterior structures. UBM is useful in measuring corneal pachymetry, especially prior to refractive surgery or in cases of corneal edema or ectasia. Since frequency is too low for the tissues to absorb, there is no tissue damage and the transmissions are instead reflected.25
While UBM technology is typically affordable and portable, the high level of technical skills needed to operate it limits its use. Additionally, the patient needs to be supine and the device must be in contact with the eye. Environmental factors like room illumination and lighting also affect the anterior segment, so these factors must be held constant when analyzing the image.25
In Vivo Confocal Microscopy (IVCM)
IVCM is a noninvasive procedure that provides a high-resolution real-time analysis of living corneal tissues at the cellular level. Whereas with a conventional light microscope the entire specimen is evenly illuminated from a light source and the depth seen is limited to that at which light penetrates into the tissue, IVCM only uses one pinpoint source of light and images the one point of the specimen, allowing for high-resolution imaging even through opaque objects. Multiple images are then acquired through rapid scanning and then reconstructed digitally for three-dimensional images of the specimen’s interior structures.
There are several different types of IVCM technology, including tandem-scanning, scanning-slit and laser scanning confocal microscopes. Tandem-scanning IVCM provides high corneal endothelium images, but isn’t as effective for imaging specific structures like the organelle within cells of the cornea.26 Scanning-slit confocal microscopes can provide high-resolution images at the cellular level, but the photos are limited to transparent tissue. Laser scanning confocal microscopy allows for high-resolution images at the cellular level including that of opaque tissue.
In vivo confocal microscopy has been able to identify and visualize epithelial cells and endothelial cells in a variety of depths of the cornea.26 Pathological abnormalities of the cornea at a microstructural level have been examined in patients with ocular rosacea, Sjögren’s syndrome, dry eyes, Stevens-Johnson syndrome and keratitis.27 IVCM is currently considered the most optimal modality at the moment that can evaluate and capture corneal nerves in vivo with high resolution.26
| Clinical Pearls • Though slower and more expensive than corneal topographers, corneal tomographers are also noncontact and are capable of producing three-dimensional images of the entire anterior segment of the eye. They also allow for pachymetry measurements. • Ultrasound biomicroscopes require contact of a probe on the patient’s eye, but are capable of imaging corneal details at the the cellular level. • Confocal microscopy can evaluate corneal nerves in vivo. • Though all of the above devices individually are very reliable and repeatable, reciprocity of results may not always be in agreement when interchanging between devices in comparison studies because each manufacturer uses their own algorithms to produce their results.28-35 |
So, How Do You Choose?
There have been many studies that compare accuracy and repeatability of various diagnostic imaging devices. Though all of the above devices individually are very reliable and repeatable, reciprocity of results may not always be the same when interchanging between devices in comparison studies because each manufacturer uses its own algorithms to produce their results.28-35 One study comparing Scheimpflug imaging devices found that while they all worked adequately, the measurements of anterior keratometry and posterior keratometry achieved with the Pentacam were significantly better than those of Galilei and Sirius in keratoconic eyes.36 A second study also comparing three Scheimpflug devices (the Galilei G2 Dual Scheimpflug Analyzer, Pentacam HR and Sirus) determined inter-device repeatability and reproducibility, but noted that maximum anterior and posterior corneal elevation and total high order aberrations from the Sirus 3D and Galilei G2 were not interchangeable with the Pentacam HR’s results.29
Meanwhile, another study comparing an AS-OCT instrument (Casia) and a rotating Scheimpflug camera with Placido topography system (TMS-5) found measurements of keratoconic patients using the former were more repeatable.37 Also, a comparison of a corneal topographer (Orbscan) and a rotating Scheimpflug imaging system (Pentacam) found that the topographer is capable of evaluating thinner corneas in keratoconic eyes and those with corneal opacities.12
Overall however, it is difficult to compare different comparison studies since each study uses unique methodologies to evaluate repeatability.28 Each imaging device has its own unique advantages and limitations, and instrument selection depends upon the clinical needs of the practice. Because many of the devices are not interchangeable, accurate clinical interpretation is required to augment the plethora of information from each of these devices. Nevertheless, the imaging devices can provide a wealth of information to supplement traditional slit lamp examinations.
| Imaging Device | Type | Advantages | Disadvantages |
| Corneal Topography | Placido disc |
|
|
| Corneal Topography | Color LED |
|
|
| Corneal Tomography | Slit Scanning |
|
|
| Corneal Tomography | Scheimpflug Imaging |
|
|
| Scheimpflug-Based Noncontact Tonometry |
|
| |
| Anterior Segment OCT | Time Domain |
|
|
| Anterior Segment OCT | Spectral Domain |
|
|
| Ultrasound Biomicroscopy |
|
| |
| Confocal Microscopy |
|
|
Dr. Yeung is the senior optometrist at the UCLA Arthur Ashe Optometry clinic and a clinical assistant professor at Western University Health Sciences College of Optometry.
Brian Kit is a class of 2016 pre-optometry UCLA undergraduate majoring in microbiology, immunology and molecular genetics.
1. Timoney PJ, Breathnach CS. Allvar Gullstrand and the slit lamp 1911. Ir J Med Sci. 2013;182(2):301-5.
2. Klein SA. Axial curvature and the skew ray error in corneal topography. Optom Vis Sci. 1997;74(11):931-44.
3. Goto T, Zheng X, Klyce SD, et al. A new method for tear film stability analysis using videokeratography. Am J Ophthalmol. 2003;135(5):607-12.
4. Kojima T, Ishida R, Dogru M, et al. A new noninvasive tear stability analysis system for the assessment of dry eyes. Invest Ophthal Vis Sci. 2004;45(5):1369-74.
5. Nemeth J, Erdelyi B, Caskany B, et al. High-speed videotopographic measurement of tear film build up time. Invest Ophthalmol Vis Sci. 2002;45(6):1783-90.
6. Vos FM, Groen FCA, van Stokkum IHM, et al. A new instrument to measure the shape of the cornea based on pseudorandom color coding. IEEE Trans Instrum Meas. 1997;46(4):794-7.
7. Arni V, Sicam P, Van Der Heijde RG. Topographer reconstruction of the non-rotation symmetric anterior corneal surface features. Optom Vis Sci. 2006;83(12):910-8.
8. Kanellopoulos AJ, Asimellis G. Clinical correlation between Placido, Scheimpflug and LED color reflection topographies in imaging of a scarred cornea. Case Rep Ophthalmol. 2014;5(3):311-7.
9. Hidalgo IR, Rozema JJ, Dhubhghaill SN, et al. Repeatability and inter-device agreement for three different methods of keratometry: Placido, Scheimpflug and color LED corneal topography. J Ref Surg. 2015;31(3):176-81.
10. Kanellopoulos AJ, Asimellis G. Color light-emitting diode reflection topography: validation of keratometric repeatability in a large sample of wide cylindrical-range corneas. Clin Ophthalmol. 2015;9:245-52.
11. Shetty R, Nuijts RM, Nicholson M, et al. Cone location dependent outcomes after combined topography-guided photorefractive keratectomy and collagen cross linking. AJO. 2014;159(3):419-25.
12. Oliveira CM, Ribeiro C, Franco S. Corneal imaging with slit scanning and Scheimpflug imaging techniques. Clin Exp Optom. 2011;94(1):33-42.
13. Ambrosio R Jr, Nogueira LP, Caldas DL, et al. Evaluation of corneal shape and biomechanics before LASIK. Int Ophthal Clin. 2011;51:11-38.
14. Shah S, Laiquzzaman M, Yeung I, et al. The use of the ocular response analyzer to determine corneal hysteresis in eyes before and after excimer laser refractive surgery. Cont Lens Ant Eye. 2009;32:123-8.
15. Frings A, Linke S, Bauer E, et al. Effects of laser in situ keratomileusis (LASIK) on corneal biomechanical measurements with the Corvis ST tonometer. Clin Ophthalmol. 2015;9:305-11.
16. Zhou S, Wang C, Cai X, et al. Optical coherence tomography and ultrasound biomicroscopy imaging of opaque corneas. Cornea. 2013;32(4):e25-30.
17. Wylegala E, Teper S, Nowinsaka A, et al. Anterior segment imaging: Fourier-domain optical coherence tomography versus time-domain optical coherence tomography. J Cataract Refract Surg. 2009;35(8):1410-4.
18. Li H, Leung CK, Wong L, et al. Comparative study of central corneal thickness measurement with slit-lamp optical coherence tomography and Visante optical coherence tomography. Ophthalmol. 2008;115(5):796-801.
19. Vajzovic L, Karp C, Haft P, et al. Ultra high-resolution anterior segment optical coherence tomography in the evaluation of anterior corneal dystrophies and degenerations. Ophthalmol. 2011;118(7):1291-6.
20. Martin R, Izquierdo M, Saber A. Investigation of posterior corneal curvature in contact lens induced corneal swelling. Cont Lens Ant Eye. 2009;32:288-93.
21. Kymionis GD, Grentzelos MA, Plaka AD, et al. Correlation of the corneal collagen cross-linking demarcation line using confocal microscopy and anterior segment optical coherence tomography in keratoconic patients. Am J Ophthalmol. 2014;157(1):110-5.
22. De Benito-Llopis L, Mehta JS, Angunawela RI, et al. Intraoperative anterior segment optical coherence tomography: a novel assessment tool during deep anterior lamellar keratoplasty. Am J Ophthalmol. 2014;157(2):334-41.
23. Demirci H, Steen DW. Limitations in imaging common conjunctival and corneal pathologies with Fourier-domain optical coherence tomography. Middle East Afr J Ophthalmol. 2014;21(3):220-4.
24. Humeric V, Kucukevciloglu M. Ultrasound biomicroscopy confirmation of corneal overriding due to improper suturing of full-thickness corneal laceration. Arg Bras Oftalmol. 2014 Nov-Dec;77(6):392-4.
25. Ishikawa H, Schuman J. Anterior segment imaging: ultrasound biomicroscopy. Ophthalmology Clinics of North America, 2004 Mar;17(1):7-20.
26. Cortes DE, Strom AR, Thomasy SM, et al. In vivo ocular imaging of the cornea of the normal female laboratory beagle using confocal microscopy. Veterinary Ophthalmol. Mar 2015 [epub].
27. Bouheraoua N, Jouve L, Mohamed SE. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci. 2014 28;55(11):7601-9.
28. Wang Q, Savini G, Hoffer KJ, et al. A comprehensive assessment of the precision and agreement of anterior corneal power measurements obtained using 8 different devices. PLoS One. 2012;7(9):e45607.
29. Hernadez-Camerena JC, Chirinos-Saldana P, Navas A, et al. Repeatability, reproducibility, and agreement between three different Scheimpflug systems in measuring cornea and anterior segment biometry. J Refract Surg. 2014;30(9):616-21.
30. Kawamorita T, Nakayma N, Uozato H. Repeatability and reproducibility of corneal curvature measurements using the Pentacam and Keratron topography systems. J Refract Surg. 2009;25(6):539-44.
31. Shah J, Han D, Htoon HM, et al. Intraobserver repeatability and interobserver reproducibility of corneal measurements in normal eyes using an optical coherence tomography-Placido disk device. J Cataract Refract Surg. 2015;41(2):372-81.
32. Visser N, Berendschot T, Verbakal F, et al. Comparability and repeatability of corneal astigmatism measurements utilizing different measurement technologies. J Cataract Refract Surg. 2012;38(10):1764-70.
33. Correa-Perez M, Olmo N, Lopez-Miguel A. Dependability of posterior-segment spectral domain optical coherence tomography for measuring central corneal thickness. Cornea. 2014;33(11):1219-24.
34. Wong MW, Shukla A, Munir W. Correlation of corneal thickness and volume with intraoperative phacoemulsification parameters using Scheimpflug imaging and optical coherence tomography. J Cataract Refract Surg. 2014;2067-75.
35. Huang J, Ding X, Savini G. Central and midperipheral corneal thickness measured with Scheimpflug imaging and optical coherence tomography. Plosone. 2014;9(5):e98316.
36. Shetty R, Arora V, Jayadev C, et al. Repeatability and agreement of three Scheimpflug based imaging systems for measuring anterior segment parameters in keratoconus. Ophthalmol Vis Sci. 2014;55:5263-8.
37. Fukuda S, Beheregaray S, Hoshi S, et al. Comparison of three-dimensional optical coherence tomography and combining a rotating Scheimpflug camera with a Placido topography system for forme fruste keratoconus diagnosis. Br J Ophthalmol. 2013;97:1554-9.


