 here are significant challenges with developing new formulations for topically applied eye drops. Perhaps the biggest obstacle is finding a way to increase substantivity (dwell time on the eye) for better drug absorption. Conventional water-based lubricating drops are limited: They use water-soluble molecules in the solution and approximately 80% of the drop is eliminated via the nasolacrimal duct two minutes after instillation.1
here are significant challenges with developing new formulations for topically applied eye drops. Perhaps the biggest obstacle is finding a way to increase substantivity (dwell time on the eye) for better drug absorption. Conventional water-based lubricating drops are limited: They use water-soluble molecules in the solution and approximately 80% of the drop is eliminated via the nasolacrimal duct two minutes after instillation.1
Excipients with either increased viscosity or bioadhesive properties are commonly used to increase this dwell time. These excipients include carbopol gels, cellulose derivatives, detran, gelatin glycerin, polyethylene glycol, poloxamer 407, polysorbate 80, propylene glycol, polyvinyl alcohol and polyvinyl pyrrolidone. These agents, however, have the potential disadvantage of blurred vision, difficulty dispensing from the bottle tip and limiting pharmaceutical mixtures to water-soluble drugs.
Oil-in-water emulsions have been investigated as a vehicle to improve bioavailability of lipophilic drugs to the ocular surface. Nanoemulsions provide a high encapsulation rate, enhance stability of lipophilic drugs and increase ocular penetration. In 2002, Restasis (cyclosporine ophthalmic, Allergan), an anionic emulsion of cyclosporine A, was the first marketed ophthalmic emulsion product.2
The Cationic Alternative
Cationic nanoemulsions will interface with the negatively charged corneal cells and can be used to bring lipids to stabilize the tear film, interact electrostatically with mucins and improve ocular absorption. Compared to anionic surfactants, cationic surfactants may be toxic to the ocular surface.
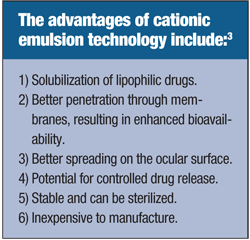 Quaternary ammoniums, such as benzalkonium chloride (BAK), have surfactant properties and can give a cationic charge to solutions. These agents work as a solution preservative by electrostatically binding to the negatively charged cell surface, and disrupting the cell membrane.3 While BAK works well as a preservative, it has been repeatedly shown to cause corneal epithelial toxicity. One study comparing latanoprost with BAK to a preservative-free cationic emulsion containing latanoprost, showed significantly reduced epithelial toxicity with the cationic emulsion in a rabbit model.4
Quaternary ammoniums, such as benzalkonium chloride (BAK), have surfactant properties and can give a cationic charge to solutions. These agents work as a solution preservative by electrostatically binding to the negatively charged cell surface, and disrupting the cell membrane.3 While BAK works well as a preservative, it has been repeatedly shown to cause corneal epithelial toxicity. One study comparing latanoprost with BAK to a preservative-free cationic emulsion containing latanoprost, showed significantly reduced epithelial toxicity with the cationic emulsion in a rabbit model.4
Cationic emulsions have better spreading coefficients across the cornea and conjunctiva vs. conventional eye drops and anionic emulsions.5 Improved spreading coefficient leads to better ocular surface wettability, and electrostatic attraction reduces tear washout. In oil emulsions, the electro-attractive interactions between the positively charged oil droplets of the cationic emulsion and the negatively charged ocular surface effectively lowers the contact angle by 50%, compared to anionic emulsions. A low contact angle, better spreading coefficient and increased dwell time of the cationic emulsion will contribute to better drug absorption of lipophilic drugs.6
On the Market Today
Cationorm (Novagali Pharma)—marketed in the United States as Retaine MGD (Ocusoft)—is the first cationic emulsion technology introduced to the market specifically for treatment of dry eye. Retaine MGD is a preservative-free, hypotonic, oil-in-water emulsion based on the positively charged emulsion and the negatively charged epithelial surface. Clinical studies have shown this product to improve spreading on the ocular surface, lengthen retention time, and reduce the number of drops needed throughout the day (which increases compliance) in both meibomian gland dysfunction and dry eye sufferers. Retaine MGD is packaged in 30 single-dose vials.
The introduction of the first cationic emulsion is a key tool for practitioners to help patients better absorb drug treatments and manage conditions like dry eye.
1. Shell JW. Ophthalmic drug delivery systems. Surv Ophthalmol. 1984 Sep-Oct;29(2):117-28.
2. Allergan. Restasis package insert.
3. Lallemand F, Daull B, Benita S, et al. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J Drug Deliv. 2012;2012:604204. Epub 2012 Feb 27.
4. Liang H, Baudouin C, Faure MO, et al. Comparison of the ocular tolerability of latanoprost cationic emulsion versus conventional formulations of prostaglandins: an in vivo toxicity assay. Mol Vis. 2009 Aug 25;15:1690-9.
5. Tiffany JM. Measurement of wettability of the corneal epithelium. II. Contact angle method. Acta Ophthalmol (Copenh). 1990 Apr;68(2):182-7.
6. Klang S, Abdulrazik M, Benita S. Influence of emulsion droplet surface charge on ocular distribution. Pharm Dev Technol. 2000;5(4):521-32.


