Dry eye disease (DED) is one of the most pervasive eye conditions in society today, affecting an estimated five million Americans age 50 or older. Of these, more than three million are women and more than 1.5 million are men.1,2 These numbers increase in the presence of systemic illnesses like Sjögren’s syndrome and rheumatoid arthritis, the use of certain medications and a history LASIK or other corneal surgery.
With this frequency, however, comes the risk that other, less common diseases can be misdiagnosed as dry eye, forcing the patient to undergo a potentially unnecessary regimen while simultaneously delaying treatment for the real problem.
When the complex tear film’s homeostasis with the ocular surface is compromised, signs and symptoms of ocular surface disease ensue. Ocular irritation, eye redness, tearing, burning and even itch are all common symptoms of dry eye, ocular surface disease and meibomian gland dysfunction. Symptoms can wax and wane, and often patients exhibit minimal clinical signs to account for their reported ocular and visual discomfort.
The Canadian Association of Optometrists recently released a comprehensive set of practical guidelines for the screening, diagnosis and management of dry eye disease.3 Interestingly, the authors point out the ocular symptoms of DED can occur as a result of many conditions, and caution practitioners to rule out alternate pathologies that may mimic or even aggravate DED. These include: allergic ocular disease, epithelial basement membrane dystrophy, conjunctivochalasis, superior limbic keratoconjunctivitis, lid issues (e.g., entropion, ectropion, lagophthalmos, floppy eyelid syndrome), computer vision syndrome, ocular cicatricial pemphigoid and others. This article will review some of the more common, but often overlooked, clinical concerns with respect to how they clinically appear similar to dry eye disease.
| |
|
|
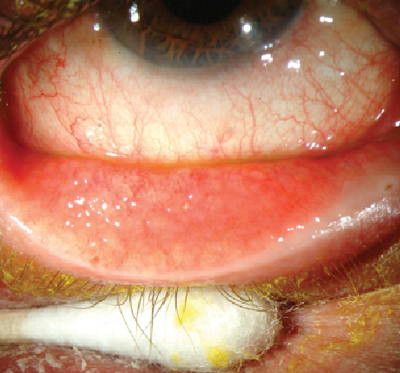
Fig. 1. Allergic conjunctivitis. Photo: Christine W. Sindt, OD |
Allergic Eye Disease
Ocular allergy is one of the most common ocular conditions worldwide, with an estimated 20% to 25% of the population suffering from some form of allergic eye symptoms.4
Other studies show the percentage to be higher in the pediatric population.5
There are six recognized allergic eye diseases. Seasonal (SAC) and perennial allergic conjunctivitis (PAC), the two most common, are IgE-mediated, hypersensitivity conditions caused by exposure to allergens, including pollen, mold and/or pet dander. Acute allergic conjunctivitis (AAC) occurs when a large quantity of an allergen inoculates the eye.6 Vernal (VKC) keratoconjunctivitis, atopic keratoconjunctivitis (AKC) and giant papillary conjunctivitis (GPC) are less common and associated with a larger amount of T-cells.6
Characteristic ocular findings associated with allergic conjunctivitis include conjunctival swelling and erythema, tearing, eyelid swelling and lower eyelid venous stasis (i.e., rings under the eyes known as "allergic shiners”).7 Some signs and symptoms of both dry eye and allergic conjunctivitis can be more subjective and vague, and may overlap. The hallmark symptom of allergic conjunctivitis, however, is itching. If the lid margins are affected more so than the globe, consider Demodex as a potential cause instead of an allergenic response.
Despite their similar symptoms, however, dry eye and allergy each has a distinctive pathophysiology: in dry eye disease, tear hyperosmolarity can develop due to either low aqueous flow or excessive tear film evaporation, triggering an inflammatory cascade that compromises the epithelium and disrupts tear film.8 Conversely, in allergic conjunctivitis, allergens cause IgE-mediated degranulation of mast cells, triggering a cascade of allergic and inflammatory mediators, such as histamine.9
|
|
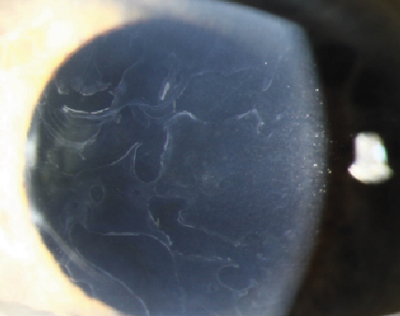
| Fig. 2. Epithelial basement membrane dystrophy. Photo: Christine W. Sindt, OD |
|
Epithelial Basement Membrane Dystrophy
Corneal dystrophies are rare, inherited corneal diseases that typically present bilaterally and asymmetrically in the first few decades of life. These hereditary disorders can be broken into three categories based on location: anterior, stromal and posterior, each with their own subcategories.
Epithelial basement membrane dystrophy (EBMD)—also known as map-dot-fingerprint dystrophy or Cogan’s dystrophy—is considered the most common corneal dystrophy, affecting roughly 76% of the global population over age 50.10 In normal circumstances, the corneal epithelium produces and adheres to its underlying basement membrane. Sometimes, however, extraneous material is produced, causing the basement membrane to thicken, become uneven and protrude into the epithelium.
Patients with EBMD typically present with transient blurred vision, photophobia, foreign body sensation and, if severe, recurrent corneal erosion (RCE); however, some patients may appear entirely asymptomatic. Definitive clinical signs of EBMD that can be observed using a slit lamp include corneal epithelial microcysts often called “dots,” whorling defects known as "fingerprints” and positive and negative sodium fluorescein staining.11 Manual keratometry can assist with making a diagnosis, as the mires appear irregular in patients with EBMD.
Other clinical corneal surface and tear film clues also exist: for instance, observing how inter-blink dynamic changes in the tear film impact the corneal reflection in the keratometer can help decipher clinical complaints. I generally recommend manual keratometry be performed on all patients, especially pre-surgery patients and those with ocular surface disease.
Typically, symptoms of EBMD are worse in the morning, as underlying dryness can cause the upper lid to stick to the poorly adhered epithelial cells and cause erosions upon awakening. Thus, it's important to remember dryness can be a comorbidity of EBMD rather than the primary condition.
Conjunctivochalasis
This fairly common age-related eye condition is characterized by the presence of redundant conjunctival folds between the eye and eyelid that develop as a result of a weakening in Tenon’s capsule. These excess folds can cover the punctal opening, mechanically interfering with tear distribution. Primary symptoms of conjunctivochalsis include dryness, redness, eye pain, foreign body sensation, blurred vision and epiphora; the dryness, redness and blurry vision are typically worse during downgaze and blinking.12,13
Risk factors for conjunctivalchalasis include: advanced age (i.e., older than 50); prior ocular surgery; and a history of dry eye or conjunctival chemosis due to longstanding allergic conjunctivitis, trauma or inflammatory conditions such as episcleritis. There may also be a connection between conjunctivochalasis and autoimmune thyroid disease: a prospective study in 2006 found that the prevalence of conjunctivochalasis in patients with autoimmune thyroid eye diseases was as high as 88%.14
Dry eye conditions can be exacerbated by the coexistence of conjunctivalchalasis, making the distinction of the two conditions more difficult. Typically, symptoms of conjunctivochalasis-induced dry eye remain steady throughout the day and worsen with vigorous blinking, while symptoms of aqueous tear deficiency dry eye appear worse later in the day and improve with blinking.15 A helpful differential diagnosis table can be found on the Ocular Surface Foundation's website (www.osref.org/medical-education-materials/conjunctivochalasis.aspx).
|
|
|
|
|
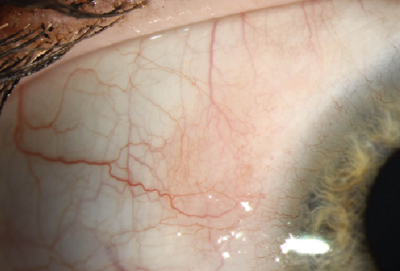
Fig. 1. Superior limbic keratoconjunctivitis. Photo: Christine W. Sindt, OD |
Superior Limbic Keratoconjunctivitis
This chronic bilateral inflammation of the superior bulbar conjunctiva and limbus typically presents with adjacent epithelial keratitis and papillary hypertrophy of the upper tarsal conjunctiva.
Patients with SLK generally complain of burning and irritation, especially during upgaze, and often experience alternating periods of remission and exacerbation with no identifiable pattern. Moisturizing medications such as artificial tears are usually prescribed, but often provide only minimal symptomatic relief.
The cause of SLK remains for the most part unknown, but it is believed to be present secondary to superior bulbar conjunctiva laxity, which induces an inflammatory response as a result of mechanical soft-tissue microtrauma from the eyelid or a contact lens.16-18 This laxity creates poor wetting of the ocular surface superiorly, leading to symptoms similar to dry eye.
Chronic inflammation from this abrasive rubbing can lead to thickening of the conjunctiva and keratinization, which further induces an inflammatory response.19 Eventually, a filamentary response may be induced on the affected cornea. Patients with corneal filaments are usually extremely symptomatic. Corneal involvement may induce scarring, astigmatism and ultimately decreased vision.
Factors thought to increase the possibility of developing conjunctival laxity include tight upper eyelids and prominent globes.20 Interestingly, SLK is also associated with thyroid dysfunction.21 Other potential risk factors for development of SLK include prolonged eyelid closure with associated hypoxia or reduced tear volume, as well as morphologic or functional changes in superior conjunctival apposition to the globe following upper eyelid procedures.22 Thus, it is recommended that every dry eye work-up should include examination of the upper bulbar conjunctiva and eversion of the upper lids to detect SLK or any other abnormalities.
Eyelid Issues
There are a number of lid-related concerns that can precipitate a dry eye state, most of which are related to exposure or mechanical stress. Graves' disease, trauma, lid or conjunctival lesions, surgery and nerve palsies are examples of issues that can lead to incomplete lid closure, compromising the interaction of the eyelid and ocular surface.23
The delivery of meibum from meibomian glands to the lid margin depends on the movement of the lids during blinking. Meibomian gland lipids are transported within the gland by the force of secretory pressure from continuous secretion and by muscular action of the orbicularis muscle and Riolans muscles during blinking.24 Deliberate, forceful blinking in particular increases the lipid layer thickness of the tear film.25 In contrast, inefficient blinking is associated with poor maintenance of lipid layer integrity.26
Thus, reduced and/or incomplete blinking along with increased tear film break-up during normal visual tasks may explain ocular discomfort symptoms, particularly at the end of the day in normal and dry eye patients.27
Computer Vision Syndrome
A recent study by the Vision Council found that, on average, roughly 93.3% of adults spend more than two hours per day using a digital device, while approximately 60.8% spend more than five hours per day.28
This frequency of use can lead to visual symptoms of computer vision syndrome (CVS) that can include: eyestrain, headaches, ocular discomfort, dry eye, diplopia and blurred vision either at near or distance.
The reduced blink rate that is common during prolonged periods of device use leads to inadequate ocular surface wetting that the patient will experience as symptoms of dryness. In CVS cases, symptoms are likely to resolve or at least lessen with reduced use of devices, whereas true cases of primary dry eye would typically persist. Discuss with patients the importance of proper ergonomics, taking visual breaks and maintaining a normal blink rate.
|
|
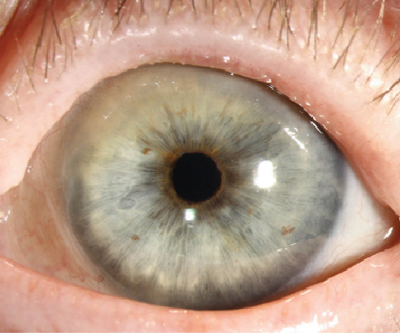
| Fig. 4. Ocular cicatricial pemphigoid. Photo: Christine W. Sindt, OD |
|
Ocular Cicatricial Pemphigoid
This chronic, progressive inflammatory disease of presumed autoimmune etiology affects the skin and mucous membranes (e.g., oral mucosa, pharynx, larynx, trachea, esophagus, vagina, urethra and anus).29
Ocular cicatricial pemphigoid (OCP) is part of a spectrum of disorders termed mucous membrane pemphigoid; it presents with a variable ophthalmic clinical picture, which includes recurrent conjunctivitis, episodes of conjunctival hyperemia and mucous discharge.30
The majority of patients with OCP are between 60 and 70 years old; however, patients in their thirties have presented with this disease, suggesting the exact age of onset may be lower, with most early-stage patients remaining undiagnosed until later.31 It predominantly affects women more often than men, with a female to male ratio of nearly 2:1.32
OCP is characterized by early onset of symptoms of monocular or binocular conjunctivitis, including irritation, burning, tearing, mucoid discharge and hyperemia. As the condition progresses, the conjunctivae undergo considerable shrinkage and fibrosis, leading to symblepharon, ankyloblepharon, xerophthalmia, keratoconjunctivitis sicca, entropion and trichiasis formation. This results in ocular surface disfigurement with corneal compromise, further leading to corneal ulceration, infection and ultimately blindness.29
OCP’s effects on mucous membranes prompts signs and symptoms of dryness that make this condition a potential mimicker of dry eye disease, but the occurence of more widespread involvement of conjunctival tissue in OCP will make the etiology apparent.
As eye care professionals, we must be aware of the signs and symptoms of ocular surface disease. Similarly, we must be sensitive to and suspicious of alternate or compounding pathology in our dry eye patients non-responsive to core ocular surface therapies. Be vigilant.
Dr. Mastrota is the center director at the New York Office of Omni Eye Services. Additionally, she serves as secretary of the Ocular Surface Society of Optometry (OSSO).
1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318-26.
2. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009;127:763-8.
3. Canadian Association of Optometrists. National Dry Eye Disease Guidelines for Canadian Optometrists. Available at: http://opto.ca/sites/default/files/resources/documents/cjo_dry_eye_supplement_2014.pdf. Accessed January 511, 2015.
4. Nathan RA, Meltzer EO, Selner JC, et al. Prevalence of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99:S808–S814.
5. Gentile D, Bartholow A, Valovirta E, Scadding G. Current and future directions in pediatric allergic rhinitis. J Allergy Clin Immunol: In Practice. 2013;1(3):214-226.
6. Buckley RJ. Allergic eye disease--a clinical challenge. Clin Exp Allergy. 1998;28 Suppl 6:39-43.
7. Hom MM, Bielory L. The anatomical and functional relationship between allergic conjunctivitis and allergic rhinitis. Allergy Rhinol (Providence) Fall. 2013;4(3):e110-9.
8. American Journal of Managed Care. Dry Eye Disease: Pathophysiology, Classification and Diagnosis. Avalable at: www.ajmc.com/publications/supplement/2008/2008-04-vol14-n3Suppl/Apr08-3141pS079-S087. Accessed February 5, 2015.
9. Ono SJ and Abelson MB. Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. Journal of Allergy and Clinical Immunology. 2005;115(1):118-122.
10. Werblin TP, Hirst LW, et al, Maumenee IH. Prevalence of map-dot-fingerprint changes in the cornea. Br J Ophthalmol. 1981; 65(6):401–409.
11. Kobayashi A, Yokogawa H, Sugiyama K. In vivo laser confocal microscopy findings in patients with map-dot-fingerprint (epithelial basement membrane) dystrophy. Clin Ophthalmol. 2012;6:1187-90.
12. Di Pascuale MA, Espana EM, Kawakita T, et al. Clinical Characteristis of conjunctivochalasis with or without aqueous tear deficiency. Sc Br J Ophthalmol. 2004;88(3):388-92.
13. Balci O. Clinical characteristics of patients with conjunctivochalasis. Clin Ophthalmol. 2014;28(8):1655-60.
14. di Almeida SF, de Sousa LB, Vieir LA, Chiamollera MI, Barros Jde N. Clinic-cytologic study of conjunctivochalasis and its relation to thyroid autoimmune diseases: prospective cohort study. Cornea. 2006;25(7):789-93.
15. Ocular Surface Research & Education Foundation. Conjunctivochalasis. Available at: www.osref.org/medical-education-materials/conjunctivochalasis.aspx. Accessed February 5, 2015.
16. Cher I. Blink-related microtrauma: when the ocular surface harms itself. Clin Experiment Ophthalmol. 2003;31(3):183-90.
17. Cher I. Superior limbic keratoconjunctivitis: multifactorial mechanical pathogenesis. Clin Experiment Ophthalmol. 2000;28(3):181-4.
18. Sun YC, Hsiao CH, Chen WL, Hu FR. Overexpression of matrix metalloproteinase-1 (MMP-1) and MMP-3 in superior limbic keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2011;52(6):3701-5.
19. Nelson JD. Superior limbic keratoconjunctivitis (SLK). Eye (Lond).1989;3 (Pt 2):180-9.
20. Medscape. Superior Limbic Keratoconjunctivitis. Available at: http://misc.medscape.com/pi/iphone/medscapeapp/html/A1194578-business.html. Accessed January 11, 2015.
21. Kadrmas EF, Bartley GB. Superior limbic keratoconjunctivitis. A prognostic sign for severe Graves ophthalmopathy. Ophthalmology. 1995;102(10):1472-5.
22. Sheu MC1, Schoenfield L, Jeng BH. Development of superior limbic keratoconjunctivitis after upper eyelid blepharoplasty surgery: support for the mechanical theory of its pathogenesis. Cornea. 2007;26(4):490-2.
23. Latkany RL, Lock B, Speaker M. Nocturnal lagophthalmos: an overview and classification. Ocul Surf. 2006;4(1):44-53.
24. Knop E, Knop N, Schirra F. Meibomian glands. Part II: physiology, characteristics, distribution and function of meibomian oil. Ophthalmologe. 2009;106(10):884-92.
25. Korb DR, Baron DF, Herman JP, et al. Tear film lipid layer thickness as a function of blinking. Cornea. 1994;13(4):354-9.
26. McMonnies CW. Incomplete blinking: exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lenses. Cont Lens Anterior Eye. 2007;30(1):37-51.
27. Himebaugh NL, Begley CG, Bradley A, Wilkinson JA. Blinking and tear break-up during four visual tasks. Optom Vis Sci. 2009;86(2):e106-14
28. The Vision Council. Digital Eye Strain. Available at: https://www.thevisioncouncil.org/content/digital-eye-strain/teens. Accessed February 5, 2015.
29. Chang JH, McCluskey PJ. Ocular cicatricial pemphigoid: manifestations and management. Curr Allergy Asthma Rep. 2005;5(4):333-8.
30. Hossain P. The evil curse of ocular pemphigoid. Eye (Lond). 2011;25(9):1107-8.
31. Foster CS. Cicatricial pemphigoid. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea Volume One: Fundamentals, Diagnosis and Management. 3rd ed. Philadelphia; Mosby Elsevier; 2011:591–599.
32. Woo SBGM. Ulcerative, vesicular, and bullous lesions. In: Greenberg MSGM, Ship JA, eds. Burket’s Oral Medicine: Diagnosis and Treatment. 11. Hamilton: B.C. Decker Inc; 2008.


