In more advanced and longer-standing cases, superficial and deep peripheral corneal vascularization localized to the lesion can also form. Subjectively, patients sometimes experience a significant decrease in lens wearing comfort, a decrease in contact lens wearing time and significant awareness of the presence of the VLK lesion.
| |
|
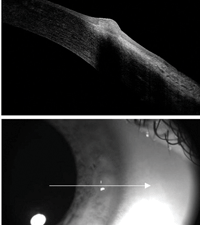
Fig. 1. VLK OD active anterior segment image.
|
VLK is often associated with gas permeable contact lens wear, in many cases, patients have a long history of GP use.1,2 The lenses that tend to trigger VLK are frequently large or steep. Additionally, lenses with low edge lift have been thought to trigger VLK. Furthermore, the lenses tend to sit centrally and have poor movement. These lens characteristics can lead to poor tear film fluid dynamics, desiccation and resulting VLK.1 Previously common PMMA contact lenses were also thought to trigger VLK if lens crazing occurred.3 Anecdotal evidence further suggests that the use of a continuous positive airway pressure machine for sleep apnea may also trigger the condition, due to dryness from air leaking into the eyes or air passing from the nose into the eyes via the nasolacrimal duct.4
Staging of VLK
VLK progresses through four stages, and patients can present in any of the four. As such, the symptoms reported by patients depend on which stage of the disease they are experiencing. Patients often report lens discomfort, lens awareness and decreased wear time. Furthermore, they may have light sensitivity, tearing and awareness of a white spot on their eye.1
Just as the symptoms of VLK depend on the stage of the condition, so too do its signs.
Stage I is characterized by a lack of symptoms, superficial punctate keratitis and epithelial hyperplasia, the latter caused by poor fluid dynamics and resulting corneal desiccation. Stage II is comprised of two phases. First, the conjunctiva becomes hyperemic. This is then followed by the possible formation of corneal infiltrates. During stage II, the patient may experience mild lens awareness and superficial punctate keratitis with staining.
Once vascularization is noted, VLK is classified as stage III. At this stage, both deep and superficial vascularization can occur, and signs of hyperemia and staining persist. Patient symptoms increase during this stage, resulting in decreased lens wearing time. Stage IV is marked by erosion of the hyperplastic area and a sharp increase in symptoms. Patients often have photophobia, pain when the lens abuts the area of elevated epithelium, and lens intolerance. Furthermore, the hyperplastic epithelium often becomes visible to the naked eye.1
Differential Diagnosis
When VLK is suspected, it is important to first rule out other conditions, such as corneal neovascularization, dellen, phlyctenulosis and pseudopterygium.1 Corneal neovascularization can be confused with stage III or IV VLK, as those are accompanied by vascularization. In corneal neovascularization, hypoxia often triggers limbal hyperemia. If the hyperemia is left untreated, it can progress to superficial neovascularization and possibly deep stromal neovascularization.
Corneal neovascularization is a rare complication of gas permeable lens wear. However, if it does occur, it often regresses with contact lens removal—leaving behind ghost vessels.9 Unlike VLK, there is no change in corneal topography and there are usually no corneal opacities. Additionally, VLK responds to treatment quicker than corneal neovascularization.1 As a result, this condition can be easily distinguished from VLK.
Much like corneal neovascularization, dellen is easily differentiated from VLK. Dellen do not have any fluorescein staining, but will pool fluorescein. Additionally, topography reveals a corneal depression instead of the staining elevation seen in VLK.1
Phlyctenulosis is another differential diagnosis to consider. Patients will often present with symptoms similar to those of VLK, and both conditions may present with a white nodule and hyperemia.10,11 VLK can be differentiated from phlyctenulosis by how quickly it regresses. VLK can regress in a few days with proper treatment; phlyctenulosis can persist for up to two weeks.1
|
|
|
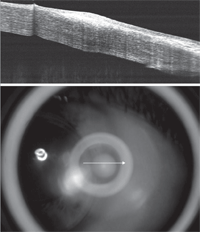
Fig. 2. Resolution of VLK following medical management and contact lens design modification.
|
Additionally, unlike VLK, phlyctenules can migrate throughout the cornea and have an infectious etiology.10
Pseudopterygia can also be cofused with VLK. Similar to VLK, it can develop as a result of gas permeable lenses drying and altering the tear film at the three and nine o’clock positions of the cornea. These signs, along with superficial punctate keratitis, can lead to vascularization extending from the limbus and eventual subepithelial opacification of the affected area.12
There are a few ways to differentiate pseudopterygia from VLK. First, a pseudopterygium only has superficial vascularization; VLK has both superficial and deep vascularization. Also, superficial punctate keratitis is found at the leading edge of the pseudopterygia, but has a diffuse presentation in VLK. Finally, pseudopterygia are more likely to leave a permanent subepithelial opacity than VLK.1
Treatment
Developing a treatment regimen for VLK depends primarily on the stage of the condition. However, changes in lens parameters enacted at any stage will help prevent future flare-ups.
Treating stage I VLK should begin by reducing lens wear time. Additionally, it may be necessary to flatten the peripheral curve to help prevent progression to further stages. In stages II-IV, it is recommended that patients discontinue lens wear entirely.1 Lenses should not be worn again until elevation and vessels have receded.2 To prevent peripheral corneal damage and improve tear dynamics, flatten the base and peripheral curves, and reduce lens diameter before the patient resumes lens wear.1,5 If the patient is wearing lenses made of silicone acrylate, it is recommended to switch to fluoro-silicone acrylate material.6
Educating patients on thorough lens cleaning techniques will also help to ensure improved wettability and tear movement.7 Alternatively, switching to a lens that vaults the cornea (e.g., prosthetic replacement of the ocular surface system or a scleral lens), will help to eliminate further corneal irritation.8 Additionally, refitting VLK patients in a soft or hybrid lens will also typically address the problem. Failure to change the lens design will likely result in a rebound of the condition.1 Upon resuming lens wear, extended wear should be eliminated.2
Along with changes in lens parameters, it is important to treat any flare-ups with the appropriate topical therapy. It is recommended to treat patients presenting with stage I or II VLK with lubricating drops. Stage III patients (and recurrences of stage II) should be treated with corticosteroids to address vascularization. Finally, due to the erosion of the cornea, it is recommended that stage IV VLK is treated with antibiotic steroid combinations.1 When treated this way, the elevated area should recede after about five days.2 If etiology is uncertain, a tissue scraping or culture may be considered in later stages.1
VLK is a relatively uncommon complication of rigid corneal contact lens wear. It typically is due to peripheral desiccation and secondary inflammation that results in a localized area of limbal hypertrophy with potential corneal infiltration, vascularization and epithelial erosion. Management includes changes to contact lens design or modality along with appropriate medical therapy. The case presented documents the appearance and resolution of VLK using anterior segment OCT.
|
Case Example
|
|
A 42-year-old white female
recently presented with common signs and symptoms of VLK. She complained
of ocular irritation OD when wearing her gas permeable contact lenses
and the presence of a small white spot on the colored part of her eye.
Her pain resolved when her contact lenses were removed. She reported
previously using Zylet eye drops (loteprednol etabonate 0.5% and
tobramycin 0.3%, Bausch + Lomb) for similar occurrences in the past.
Upon exam, vascularization and associated scarring were noted at the three and nine o’clock positions in both corneas. Additionally, a raised, circular and opaque area was seen nasally in the right eye. Fluorescein showed 3+ staining on the elevated area OD and trace superficial punctate keratitis nasally and temporally OS. The contact lens OD was seen to abut against the elevated area. An anterior segment OCT revealed significant epithelial and stromal hypertrophy nasally OD (Figure 1) and mild stromal hypertrophy nasally and temporally OS. The patient’s right eye was classified as stage IV VLK, as an eroded area of epithelial hyperplasia was noted. Surprisingly, the patient was not sensitive to light and could tolerate a small amount of lens wear. The patient’s left eye was classified as stage III VLK, as vascularization was noted without corneal erosion. In this eye, the patient still tolerated the lens very well. Treatment to stop the VLK flare-up and eliminate future problems included decreasing the contact lens diameter OU from 9.2mm to 8.7mm along with an increase in axial edge lift of the lenses. The diameter reduction and increase in edge lift prevented further corneal irritation of the affected areas. She was also prescribed Zylet QID OD for five to seven days to reduce the inflammation. At the follow-up visit, anterior segment OCT revealed a significant reduction in the stromal hypertrophy OD (Figure 2). The patient reported that the pain in her right eye had subsided and the new lenses were comfortable. Upon slit-lamp observation, the new, smaller diameter lens edge did not touch the previously affected three and nine o’clock positions of the cornea. Furthermore, the elevated nasal opacity in the right eye had dissipated and no longer showed any fluorescein staining. |
1. Grohe RM, Lebow KA. Vascularized limbal keratitis. International Contact Lens Clinic. 1989;16(7-8):197-209.
2. Bennett ES, Scheid T. Gas-permeable lens problems solving. In: Bennett ES, Henry VA, eds. Clinical Manual of Contact Lenses. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009: 183-208.
3. Miller WL. Rigid gas permeable surface defects associated with an isolated case of vascularized limbal keratitis. International Contact Lens Clinic, 1995;22:209-212.
4. Harrison W, Pence N, Kovacich S. Anterior segment complications secondary to continuous positive airway pressure machine treatment in patients with obstructive sleep apnea. Optometry. 2007;78(7):352-355.
5. Efron N. Contact lens complications. 3rd ed. New York: Butterworth-Heinemann; 2004.
6. Cannella A, Bonafini JA. Fluoro-silicone/acrylate materials: The second generation of gas permeable lenses. In: Bennett ET, Weissman BA, eds. Clinical contact lens practice. Philadelphia, PA: Lippincott Williams & Wilkins: 2005: 240-241.
7. Van Der Worp E, De Brabander J, Swarbrick H, Nuijts R, Hendrikse F. Corneal desiccation in rigid contact lens wear: 3-and 9-o’clock staining. Optometry & Vision Science. 2003;80(4):280-290.
8. Cressey A, Jacobs DS, Carrasquillo KG. Management of vascularized limbal keratitis with prosthetic replacement of the ocular surface system. Eye & Contact Lens. 2012;38(2):137-140.
9. Liesegang TJ. Physiologic changes of the cornea with contact lens wear. Eye & Contact Lens. 2002;28(1):12-27.
10. Tzelikis PF, Cohen EJ, Rapuano CJ. Phlyctenulosis. In: Roy FH, Fraunfelder FW, Fraunfelder FT, eds. Roy and Fraunfelder's Current Ocular Therapy. 6th ed. China: Elsevier Health Sciences; 2008;38:388-389.
11. Kanski J, Bowling B. Cornea. Clinical ophthalmology: A systemic approach. 7th ed. China: Elsevier Saunders; 2011:197.
12. Stainer GA, Brightbill FS, Holm P, Laux D. The development of pseudopterygia in hard contact lens wearers. Eye & Contact Lens. 1981;7(1):1-4.


