Silicone hydrogel (SiHy) contact lenses were designed to increase oxygen permeability so as to eliminate the hypoxic responses known to occur from wearing conventional hydrogel materials on an extended wear basis. The new standard is “more oxygen,” suggesting that a higher level of a critical Dk/t (diffusion coefficient/thickness) of contact lenses is required to avoid ocular surface compromise, limbal redness, corneal acidosis and to reduce central corneal edema.
It has been shown that the mean peripheral Dk/t required to avoid a change in limbal redness is 125 x 10-9 units.1 As such, the introduction of silicone hydrogel contact lenses with a higher oxygen transmission should lead to fewer complications and a reduced risk of microbial keratitis and inflammatory concerns.
The new generation of materials, SiHy lenses have been deemed clinically safer to wear for an extended period—welcome news to practitioners and patients alike.2,3 Yet, the question remains: Why are there more infiltrates found in wearers of SiHy materials than conventional HEMA?
What are Corneal Infiltrates?
Corneal infiltrates are single or multiple discrete aggregates of gray or white inflammatory cells that have migrated into the normally transparent corneal tissue.4 They are seen as small, hazy, grayish areas (local or diffuse) surrounded by edema. The infiltrates are an accumulation of polymorphonuclear leukocytes (neutrophils), lymphocytes and macrophages released from the limbal blood vessels.
Under hypoxic or stressed conditions, such as when contact lenses act as barriers for normal oxygen flow to the cornea, glucose converts to lactate, which diffuses into the stroma and results in corneal edema due to metabolic acidosis. The change in the corneal metabolism will lead to inflammation, contact lens-induced acute red eye (CLARE) and the accumulation of white blood cells that migrate from the limbal vasculature.
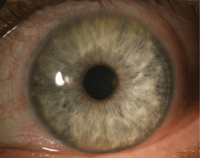
1. CLARE from extended wear SiHy lenses.
Infiltrates are often small (<2mm) and demonstrate little to no epithelial staining. These cells migrate from the limbal vasculature as a response to local tissue damage or may be associated with chemotactic factors derived from antigens and environmental toxins, or from an exogenous source such as contact lens solutions or accumulated bioburden.5 These vessels are typically located near a vascularized region of the limbus, which may also subsequently appear hyperemic (reddened).
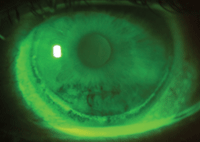
2. Circumlimbal epithelial splitting from extended wear SiHy lenses.
When Do We See Them?
Infiltrates are a consequence of the corneal inflammatory response to insult with an increased microbial bioburden; solution preservatives and hypoxic conditions are responsible for more than a 70% of the total risk of corneal infiltrates in silicone hydrogel extended wear.6
There have been suggestions that silicone hydrogel lenses, when worn on an extended wear basis, increase the relative risk of infiltrative keratitis.7 In one study, researchers cited that there was a two-fold higher risk for corneal inflammatory events with individuals who wore SiHy lenses for extended wear (30 days) as compared to individuals who wore low Dk extended wear lenses for seven continuous days.8 They could not fully link the risk to material alone.
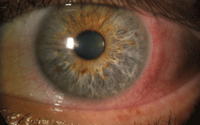
3. CLARE presenting with extreme conjunctival injection in absence of corneal infiltrates.
Another study found that there are differences in symptomatic (clinically relevant) and asymptomatic (clinical observed) cases. The incidence of symptomatic patients presented at a rate of 1.9%. Asymptomatic patients (observed in follow-up) presented at a rate of 7.2% for daily use and 26% for extended wear with use of silicone hydrogel lenses.9 During 30-night extended (continuous) wear with silicone hydrogels, the incidence of symptomatic corneal infiltrates (or ≥Grade 2) were documented to be at an incidence rate of 2.5% to ~6% per year.
In a separate meta-analysis of 23 studies, the incident rates are significantly higher with silicone hydrogel. Between 1990s until 2006, it was reported that infiltrates occurred at a variant annual rate (14.4% for silicone hydrogels and 7.7% for traditional hydrogel materials) between symptomatic and asymptomatic corneal infiltrate events.9

4. AIK with a visual complaint: Central cornea haze edema with epithelial compromise and sub-epithelial infiltrates.
Collectively, this suggests that extended wear patients require more intensive follow-up and greater surveillance than their daily wear counterparts. If infiltrates occur, it is suggestive that compromise has already occurred and intervention is clinically required.
Barring the duration of wear, there is no one specific reason for the risks of inflammatory events other than material interactions with care products and the effect on the ocular surface and flora. As discussed by Joseph Hutter, PhD, of the FDA, the classification of SiHy materials is based on the material interaction with care products and protein interactions.10,11 Solutions (surfactant, buffer or preservative agents) react differently to SiHy materials vs. conventional HEMA materials; the variance in the chemistry of the SiHy lenses may have an effect on the protein relationship that may induce an inflammatory reaction. Both HEMA and SiHy lenses have protein deposition and related material pore blockage, therefore both have the same potential for bioburden and a decrease of listed Dk/t for the specific material.
There are several relationships that have been suggested to enhance the risk factors of inflammatory infiltrates in all lenses, including silicone hydrogels. These include patient age, degree of refractive error and wear schedule, according to one study.12 The study concluded that younger patients may have increased “at risk” behavior related to compliance and hygiene issues, and a more dismissive (wait and see) health care attitude. In addition, men were substantially more dismissive of addressing health care issues than women.13 This may also be related to the presentation of symptomatic vs. asymptomatic patients.
Age might also be considered in the related bioburden issues, such as periocular and ocular surface flora leading to a stressed normality. When prolonged wear times with the developing imbalance of the bacterial homeostatic relationship is also added into the equation, the potential infiltrative response recipe increases. In older patients, there may also be lid anomalies (blepharitis or dry eye syndrome), a stressed corneal metabolism and a weakening of the strength and integrity of the corneal structure due cell loss of the endothelium over years.
The study also suggested that higher degrees of ametropia (5D or greater) had an increased risk of infiltrates even though no specific relationship was noted.12 Here, a supposition about the relationship between material and oxygen can be made. Dk/t is a measure in the central core of the material and does not relate oxygen transmissibility well to other portions of the overall lens. The peripheral architecture of the lens has a direct impact on oxygen transmissibility and the overall oxygen supply of the anterior cornea.14 A significant decrease in the overall Dk/t occurs based on the power parameter, plus centrally and higher minus in the periphery of the lens. As such, the potential benefit of SiHy oxygen transport might be substantially diminished.
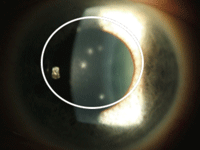
5. Viral-related infiltrates in a contact lens wearer.
Also, of note, smoking is seen to increase the risk of microbial and infiltrative disease.12 Smoking compromises the immune system while the toxins accumulate and inoculate the lenses, and dissolutes to the ocular surface over time.15 The compromised immune system and the corneal toxicity lead to a greater compromise of the ocular defense system. Other behaviors, including poor storage case hygiene, purchasing contact lenses online and changes in socioeconomics, are characteristic of poor compliance.
A Treatment Plan
When observing corneal infiltrates it is important to critically differentiate between infectious and/or sterile influences to determine a treatment course. CI can classified as either asymptomatic infiltrative keratitis, asymptomatic infiltrates, CLARE, contact lens-induced peripheral ulcer, infiltrative keratitis or microbial keratitis.16
There are many variant presentations of corneal infiltrates that can be solely inflammatory or may have an infectious etiology (see Table 1). Since all these entities have an inflammatory component, steroidal intervention is an important consideration. Note: It is critical to consider a potential infectious co-disease that may suggest concurrent antimicrobial coverage. Do not forget to rule out systemic viral associations in light of contact lens wear.
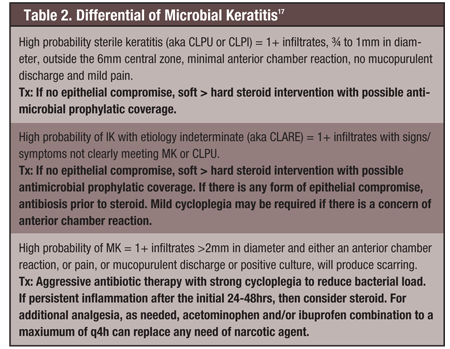
The differential diagnosis of contact lens-related infiltrative keratitis is used to determine the appropriate level of concern and to guide the clinical approach to effective management (see Table 2). While it is easy to over-treat infectious processes, the possibility of under-treatment is of greater concern, as it may exacerbate the condition. Determining whether antibiosis should be used alone and/or in combination with a steroid is always a difficult clinical decision. Always be cautious and consider antibiotic monotherapy if there is any form of epithelial compromise. Antibiosis used in conjunction with cycloplegia for 24 to 48 hours will reduce the bacterial load to a safer level, after which steroid intervention may be introduced if needed.
Silicone hydrogel lenses are touted as delivering more oxygen, with reduced hypoxia-associated complications, and they deliver on that front. But more oxygen does not always mean fewer complications. The clinical management of a patient must go beyond simply switching from HEMA to silicone materials based on the sole characteristic of oxygen transmissibility.
Remember, a contact lens is a barrier in the natural physiology of the cornea and anterior segment. Hypoxia is reduced with silicone hydrogel lenses, but the material itself can cause other compromises to the cornea due to misuse of the lens material and improper care products. Clinical awareness and management is critical in decreasing and avoiding complications. This comes down to proper patient care and education to foster the optimal use of these materials.
Kenneth Daniels is in a private practice in Hopewell and Lambertville, N.J., He also is an assistant clinical professor at Salus University in Elkins Park, Pa, and an external education preceptor for New England College of Optometry in Boston.
1. Papas E. On the relationship between soft contact lens oxygen transmissibility and induced limbal hyperaemia. Exp Eye Res. 1998 Aug;67(2):125-31.
2. Donshik PC. Extended wear contact lenses. Opthalmol Clin North Am. 2003 Mar;16(1):79-87.
3. Foulks GN. Prolonging contact lens wear and making contact lens wear safer. Am J Ophthalmol. 2006 Feb;141(2):369-73.
4. Corneal infiltrates. The Free Dictionary. Available at:
http://medical-dictionary.thefreedictionary.com/corneal+infiltrates. Accessed October 14, 2012.
5. Weissman B, Giese M, Mondino B. Ulcerative bacterial keratitis. In: Silbert J (ed). Anterior segment complications of contact lens wear. 2nd ed. Boston: Butterworth-Heinemann, 2000:225-49.
6. Szczotka-Flynn L, Lass JH, Sethi S, et al. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5421-30.
7. Sankaridurg PR, Holden BA, Jalbert I. Adverse events and infections: which ones and how many? In: Sweeney DF (ed). Silicone hydrogel: continuous-wear contact lenses. Edinburgh: Butterworth-Heinemann; 2004:217-74.
8. Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007 Apr;84(4):247-56.
9. Szczotka LB, Chalmers R. Corneal infiltrates: managing risks with soft lens wear. CL Spectrum. 2012 Jan; 27:12-3. Available at: www.clspectrum.com/printarticle.aspx?articleID=106540. Accessed November 2012.
10. Hutter J. FDA group V: is a single grouping sufficient to describe SiH performance? 2007 Nov. Available at: www.siliconehydrogels.org/editorials/nov_07.asp. Accessed December 2012.
11. Tomasi JK, Wilhelm M, Obendorf D, et al. Change of oxygen transmissibility/permeability during prolonged wear of contact lenses. Universtät Innsbuck. Available at:
http://homepage.uibk.ac.at/~c10245/dokumente/elach5.pdf. Accessed December 2012.
12. Chlamers RL, McNally J, Schein OD, et al. Risk factors of corneal infiltrates with continuous wear of contact lenses. Optom Vis Sci. 2007 Jul;84(7):573-9.
13. Bertakis KD, Azari R, Helms LJ, et al. Gender differences in the utilization of health care services. J Fam Pract. 2000 Feb;49(2):147-52.
14. Ehrmann K, Falk D, Perera I, et al. Oxygen transmissibility of various silicone hydrogel lenses. Available at:
www.siliconehydrogels.org/posters/pdf/200911.pdf. Accessed December 2012.
15. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008 Oct;115(10):1655-62.
16. Silbert JA. Corneal infiltrative complications associated with contact lens wear. Rev Optom. 2004 Apr 15. Available at:
http://cms.revoptom.com/index.asp?ArticleType=SiteSpec&Page=osc/apr04/ciba.htm. Accessed November 2012.
17. Schein O, McNally J, Katz J, et al. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Opthalmology. 2005 Dec;112(12):2172-9.


