After graduating from The Ohio State College of Optometry, I worked in a busy practice that dispensed multiple starter kits to patients. The doctors and technicians often told patients to “try each one and see which you like the best.” Looking back, I try to think about how the patient perceived that message and, more importantly, whether it helped build the doctor-patient relationship. In this article, I address the importance of open communication when reviewing contact lens solution options and the pitfalls of deviating from specific recommendations.
A Classic Scenario
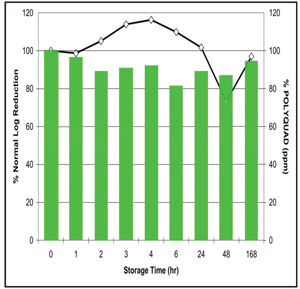
1. Fusarium disinfection efficacy over a one week storage with Polyquad based solution.
“Ms. Smith, you indicated that you wear your contact lenses for several hours each day and you want a more breathable lens. Keeping that in mind, I am prescribing these contact lenses specifically to you. You also mentioned that you have some end-of-day discomfort, so I want you to take home these four different contact lens solution trial kits and see which one feels most comfortable. If you find one you like, stick with that solution. Please call my office if you have any problems before your next visit.”
This scenario has both good and bad elements. The first part was executed perfectly. The eye care professional listened to the patient’s concerns and selected a more breathable contact lens to best meet her needs. The second part of the consultation is more problematic. By handing out multiple solution kits without properly educating the patient on each one, you have soured the patient experience. From the patient’s perspective, the eye care professional went from prescribing the proper medical device for the corneal physiology to not having an expert recommendation for the contact lens solution. The patient may perceive that lack of recommendation as either a strike against the doctor for not knowing enough to recommend a particular solution or misinterpret the message to mean that all solutions are similar and interchangeable. Even worse, the patient could leave thinking the doctor didn’t have time to properly meet her needs. It is therefore vital to properly educate the patient on lens care at each and every visit.
Listening Comes First
Contact lens care adherence, not compliance, should be the main focus with our contact lens practices (See, “Secret Shopper Finds Lack of Contact Lens Education,” Review of Optometry, November 2010). An open discussion with your patients regarding their hygiene, lens care and solution regimen is important at every visit. I suggest having a checklist that you can refer to during each patient visit.
Your contact lens agreement is a key document that should be reviewed regularly. At least once a year, you should discuss the wear/replacement schedule, proper solutions and lens care hygiene with your patient and keep a signed copy of the document in his or her file. One important point of your contact lens agreement or chart documentation should focus on educating the patient on the increased risk of microbial keratitis when using contact lenses versus spectacle-only correction.
The truth is that, when used correctly, the inherent risks for contact lens adverse events are very low. Daily wearer microbial keratitis risk is 4/10,000 or 20/10,000 for extended wear, and the risk of ulcerative keratitis in contact lens wearers who smoke is three to eight times greater than nonsmokers.1
Educate the Patient
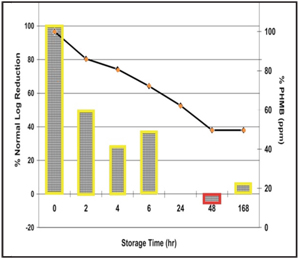
2. Fusarium disinfection efficacy over one week with PHMB based solution.
Silicone hydrogel lenses comprise 60% of the current lens market, and this statistic has almost doubled since 2005.2 When you fit a patient in a more breathable lens, let him or her know that the whites of the eyes should appear whiter and the corneas will also breathe more oxygen, halting the progression of new blood vessel growth. But don’t stop there. Let the patient know that combining these technologically advanced medical device materials with different contact lens solutions can adversely affect contact lens comfort and increase risks for adverse events, such as microbial keratitis.
It is imperative that you make a confident recommendation to the patient. Give the patient at least one reason why you are prescribing a specific solution for their lens material, lens care and eye physiology.
Understand the Risks: Lens/Material Kinetics
In 2006 and 2007 respectively, two different lens solution manufacturers had to recall their solutions. The Morbidity and Mortality Weekly Report from the CDC cited topping off and insufficient antimicrobial activity for both recalls.3,4 Yet, the FDA does not require a contact lens manufacturer to test any lens with their solution. The FDA requirements only include testing the solution against certain microbes in a test tube—the ISO 14729 Stand Alone Test. However, when you soak a lens in a lens solution, an interaction can occur between the preservative and lens material. The lens material adsorbs or “uptakes” the preservative in a process known as lens/material kinetics.5 The preservatives used in solutions are employed to kill pathogens; however, if these biocidal agents interact with the lens material, it decreases the disinfection profile of the solution originally approved by the FDA—even though no lens was used for testing.6
Figures 1 and 2 demonstrate Acuvue 2 (Vistakon) lenses soaked in the respective solutions and then challenged at different times.6 As the graph explains, the disinfection profile of efficacy against Fusarium at baseline (as reported by the manufacturer to the FDA) and the disinfection potency at the end of one week is different for the PHMB-based solution.
At the 2010 AOA conference, William Benjamin, O.D., Ph.D., of Birmingham, Ala., provided a medical device update on the new ISO 11986 standards—updated to require evaluation of the rate of uptake and release rather than just the maximum statistics. This is a huge step forward toward patient safety, and may potentially help avoid any disastrous solution recalls.17
Understand the Risks: Preservative Release
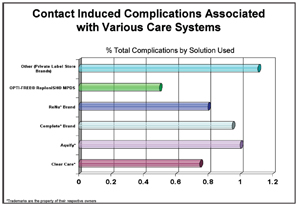
3. Complications are twice as likely with generic or store brand solutions.13
One of the more recent topics of discussion is solution-induced corneal staining (SICS). Since the first silicone hydrogel lenses were launched around 1999, many practitioners started noticing high amounts of corneal staining with certain lens/solution combinations.7 Some researchers named this phenomenon “preservative release,” when the contact lens is applied to the eye and staining is documented. SICS has been published in many different research publications and it can occur as early as two hours and remain at various levels throughout the remainder of every lens wearing day.8,9
SICS is a chronic disruption to the epithelial cell layer and research has shown it increases a contact lens wearer’s chance of a corneal infiltrative event three-fold.9 Researchers have also discovered that when combining specific lens materials with PHBM-based solutions, the subjective comfort preference decreases with growing amounts of corneal staining.10 Therefore, patient discomfort and eventual contact lens dropout may have several negative ramifications.
Comfort is the primary reason that patients decide to either continue or discontinue contact lens wear.11 If patients are not happy with their current contact lens comfort, they may seek out another eye care provider or return to exclusively wearing glasses. This is less than ideal for practitioners, as studies have shown that contact lens patients generate 60% more revenue than spectacle-only patients.16
One study even indicated that contact lens wearers generated 91% more revenue over a six-year period than spectacle wearers.12 So, it is essential to educate your patients about potential lens/material interactions that could cause corneal staining and how their comfort could be adversely affected if they decide to buy a non-recommended solution.
A UCLA study found that patients with a contact lens complication were twice as likely to use generic/store brand solution instead of the leading multipurpose branded solution13 (figure 3). Also, when prescribing a hydrogen peroxide disinfection system, make sure to discuss how to properly handle the solution to prevent toxic burns. And, take a few minutes to explain how the system works—in particular, how one-step systems neutralize hydrogen peroxide within 90 minutes. This quick neutralization process is not effective against Acanthomoeba, which may need a 3% concentration soak for four hours to effectively kill Acanthamoeba cycts.14
A Case Study
4. A silicone hydrogel lens wearing patient complained of eye redness.
A new patient came into my office about two years ago complaining of 20 pink eye infections over the past year. She gauged her level of comfort as very poor with her current contact lenses. She also explained that she had visited several medical and eye care providers complaining of eye redness, but each time, she was given a one-week topical antibiotic eye drop prescription (sometimes a combination antibiotic/steroid eye drop) and was asked to discontinue her lens use until the condition resolved.
After months of frustration, the patient finally ended up in my chair for her yearly exam and wanted an answer to her contact lens woes. I learned that she was wearing a silicone hydrogel prone to high amounts of preservative uptake/release and was using generic, “sale of the week” store brand contact lens solutions. Immediately after switching her to a different preservative-based system, her frequently occurring pink eye had resolved and her comfort level had increased dramatically.
Very few eye care professionals, if any, recommend a generic or store brand multipurpose solution. In fact, most patients purchase their contact lens solutions based on the recommendation of their eye care professional (figure 5).15
Lessons to Learn
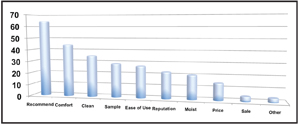
5. The reasoning behind how patients purchase contact lens solutions.15
It is our livelihood to know each solution and how to effectively match the right solution with the right patient. We should always be able to give our patients at least one solid recommendation. Remind your patients that combining different medical devices—such as a lens and solution—can be a very complex and risky experiment, particularly when the patient decides to change their lens care without first discussing it with you. Finally, remember that educated patients are willing to listen to your advice and stick with your recommendations, so make open communication a priority in your practice.
Dr. Michael Mayers is a graduate of The Ohio State University College of Optometry. He has served as a National Board Examiner and has lectured nationally on various topics. He is a fellow of the American Academy of Optometry and conducts clinical research studies in the field of contact lenses, contact lens solutions and dry eye. His office is in Powell, Ohio where he founded Mayers Eye Solutions LLC.
1. Schein OD, Poggio EC. Ulcerative keratitis in contact lens wearer. Incidence and risk factors. Cornea. 1990:9 Suppl 1:S55-8; discussion S62-3.
2. Nichols J. Contact Lenses 2009. Contact Lens Spectrum. 2010 Jan. Available at: www. clspectrum.com/article.aspx?article=103778 (Accessed October 2010).
3. Verani J, Lorick S, Yoder J, et al. National outbreak of Acanthamoeba Keratitis associated with use of a contact lens solution, United States. EID Journal. 2009 Aug; 15(8). Available at: www.cdc.gov/eid/content/15/8/1236.htm (Accessed October 2010).
4. Barry M, Pendarvis M, Mshar P, et al. Update: Fusarium Keratitis––United States, 2005-2006. CDC. 2006 May: 55(Dispatch): 1-2. Available at: www. cdc.gov/mmwr/preview/mmwrhtml/mm55d519a1.htm (Accessed October 2010).
5. Sentell K, Beaullieu E. Comparison of preservative uptake and release profiles of PHMB from soft contact lens care products by silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2004;45: E-Abstract 1573. Available at: www.abstracts.iovs.org/cgi/content/abstract/45/5/1573 (Accessed October 2010).
6. Rosenthal RA, Dassanayake NL, Schlitzer RL, et al. Biocide uptake in contact lenses and loss of fungicidal activity during storage of contact lenses. Eye Contact Lens. 2006 Dec;32(6):262-6.
7. Epstein A. SPK with daily wear of silicone hydrogel lenses and MPS. Contact Lens Spectrum. 2002;17:30.
8. Carnt N, Evans V, Holden B, et al. IER matrix update: adding another silicone hydrogel. Contact Lens Spectrum. 2008 Mar. Available at: www. clspectrum.com/article.aspx?article=101452 (Accessed October 2010).
9. Carnt N, Evans V, Naduvilath T, et al. Contact lens-related adverse events and the silicone hydrogel lenses and daily wear care system used. Arch Ophthalmol. 2009 Dec;127(12):1616-23.
10. Lebow KA, Schachet JL. Evaluation of corneal staining and patient preference with use of three multi-purpose solutions and two brands of soft contact lenses. Eye Contat Lens. 2003 Oct;29(4):213-20.
11. Rumpakis J. New data on contact lens dropouts: an international perspective. Rev Optom. 2010 Jan;147(1):37-42.
12. Practice Advancements Associates. Estimates based on CL industry audits. CIBA Vision wearer model, Jobson publishing frames and lenses estimates.
13. Forister JF, Forister EF, Yeung KK, et al. Prevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens. 2009 Jul;35(4):176-80.
14. Kilvington S, Gray T, Dart J, et al. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci. 2004 Jan;45(1):165-9.
15. Miller J, Powell S, Espejo L, et al. Solution Recommendations for Soft Contact Lens Wearers. 2010. Poster presented at American Optometry Association; Orlando, Fl.
16. Ritson M. Which Patients are More Profitable? CL Spectrum. 2006 Mar;21(3):38-40,42.
17. ANSI. Ophthalmic optics—Contact lenses and contact lens care products - Determination of preservative uptake and release. 2010. Available at: www.webstore.ansi.org/RecordDetail.aspx?sku=ISO+11986:2010. (Accessed October 2010).


