Corneal transplantation has changed dramatically since Eduard Zirm performed the first one on a human just over a century ago. As an avascular tissue lacking lymphatic vessels, the cornea enjoys immune privilege, resulting in it being associated with one of the lowest rejection rates of all human organs.1 In recent years, the evolution of techniques and technologies has substantially improved outcomes and enabled a shift toward replacement of only the diseased layers. The different transplant types can be a bit disorienting, but understanding their indications and knowing how to perform perioperative care can make a world of difference for optometrists and their patients.
Keratoplasty Indications
The two main categories of keratoplasties are full-thickness and partial-thickness. As the latter procedures only remove one or more selected layers of the cornea, they are also referred to as lamellar. These keratoplasties can be further classified as either anterior or posterior. The indications for transplantation are numerous and include optical, tectonic and reconstructive, therapeutic and, in rare cases, cosmetic needs. Patients who have a significantly reduced best-corrected visual acuity (BCVA) from a corneal pathology usually benefit from a graft. Occasionally, transplantation is required to save an altered corneal structure from perforation or thinning. Pain management for bullous keratopathy and non-healing ulcers can also be a primary therapeutic indication. Cosmetic reasons for surgery in eyes without visual potential remain controversial and are considered weak indications.
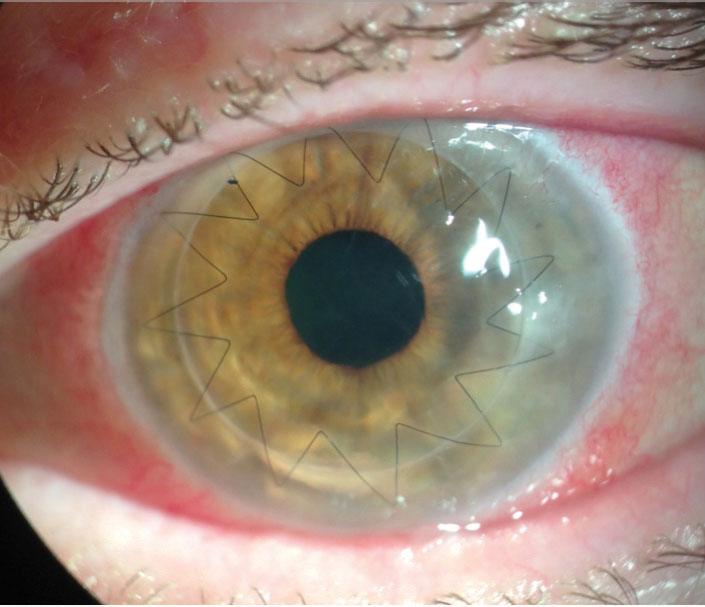 |
| Ten months after a full-thickness PKP, a 68-year-old white female developed methicillin-resistant Staphylococcus aureus keratitis at the graft-host junction and early endothelial rejection after suture removal. She was treated with fortified antibiotics, steroids and an intrastromal injection. After a week, only a small scar remained. Photo by Lawrence Tenkman, MD. |
Full- vs. Partial-Thickness
Historically, full-thickness penetrating keratoplasties (PKP) have been the primary approach to corneal transplantation, during which all layers are replaced. The technique is standardized and familiar to most corneal surgeons. However, complications are relatively common, and recovery can be painstaking and take up to a year to stabilize. Vision may actually be worse at first and not improve for six months or longer, partially due to the unpredictability of the cornea’s toricity, and often requires rigid contact lens correction. Penetrating trauma, corneal hydrops and diseases involving the stroma and endothelium still require full-thickness grafts. However, pathology involving 85% to 95% of the cornea, sparing Descemet’s membrane (DM) and the endothelium, may be best suited for a partial-thickness graft or a deep anterior lamellar keratoplasty (DALK).
Corneal ectasias, stromal dystrophies and scarring from a previous infection often qualify for a DALK, which has major advantages over a PKP. The risks of infection, hemorrhage and traumatic wound dehiscence associated with “open sky” procedures are considerably reduced. DALKs also avoid the most common and serious type of rejection—endothelial—and cause significantly less endothelial cell loss than PKPs.2 Patients who receive a DALK stabilize more rapidly, allowing for earlier suture removal and tapering of topical steroids. The surgery itself, however, is more challenging. While using the Anwar big-bubble technique to detach DM from the stromal layers aids in a faster and overall safer approach, it also increases surgical complexity and introduces other risks. Intraoperative DM tears may result in DM detachment, which is also called a double anterior chamber (AC).3
Postoperative management is similar to that of a full-thickness keratoplasty and requires a long course of topical steroids. Half of PKP patients end up with at least four diopters of astigmatism, which is often irregular and could produce significant anisometropia and aniseikonia. Unfortunately, the likelihood of high astigmatism is not lessened in a DALK, and satisfactory visual rehabilitation with rigid gas permeable lenses is often still required.
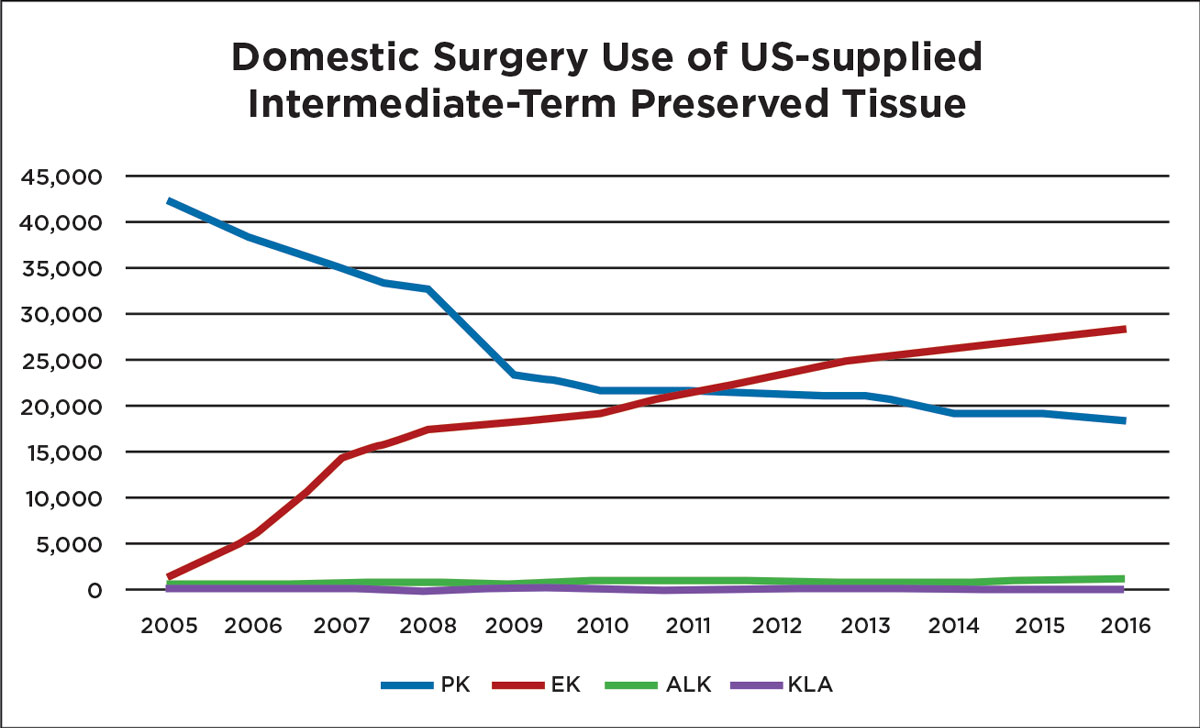 |
| Improvements in surgical technique have allowed endothelial keratoplasties to thrive in recent years, eclipsing full-thickness procedures. Image by Eye Bank Association of America. Click to enlarge. |
The Endothelial Route
Since 2011, the number of endothelial keratoplasties (EK) in the United States has surpassed full-thickness grafts.4 For posterior corneal disease, an EK is superior to a PKP or a DALK in nearly every aspect and is associated with a better visual acuity, a faster recovery time and a lower rejection rate. There are two primary types of EKs: a Descemet’s stripping automated endothelial keratoplasty (DSAEK) and a Descemet’s membrane endothelial keratoplasty (DMEK). A DSAEK graft includes some posterior stroma, DM and the corneal endothelium and comprises about 100µm to 200µm of tissue. A DMEK graft does not contain any stroma and may only be 10µm to 15µm in thickness. In 2017, the number of PKP grafts in the United States was 18,346 (a decrease of 1.3% since 2016), while EK numbers increased by 2.6% to 28,993, largely due to the 18.1% increase in DMEK procedures.5
The most common indication for an EK is Fuchs’ endothelial dystrophy (FED).6 Others include pathologies confined to the endothelium and DM, such as posterior polymorphous dystrophy and some cases of pseudophakic bullous keratopathy. FED is usually inherited as an autosomal dominant condition that causes endothelial cells to die off faster than normal. Female patients commonly suffer from a more debilitating version of the disease.
The hallmark beaten-metal appearance we refer to as guttata starts to appear during a patient’s third decade. Using a specular reflection technique when examining guttata with a slit lamp may produce the best results. Retroillumination of the iris or fundus is helpful if the beam width is moderately narrow, allowing for the appropriate contrast. Newer technologies provide endothelial cell counts via specular microscopy. The AC and corneal thickness can now be visualized with anterior segment OCT. Occasionally, guttata deposition may be so confluent that it has a whitish plaque appearance. Some corneal haze represents permanent fibrosis.
Ideally, an EK should be performed before any stromal scarring appears. Patients who notice morning worsening of their vision have started their descent toward corneal endothelial decompensation. Concentrated sodium chloride ophthalmic drops may help symptoms temporarily, but patients should be closely monitored, and transplantation should be seriously considered, especially if epithelial edema develops. Many cases, unfortunately, progress rapidly, with corneal bullae into dense subepithelial haze, possibly eliminating a patient’s candidacy for an EK.
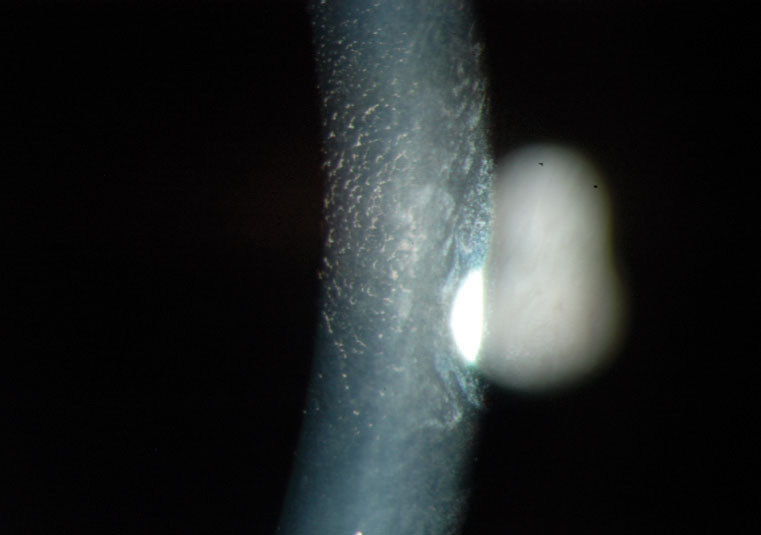 |
High magnification image of endothelial corneal guttata by specular reflection. |
New and Improved Techniques
Educating patients on surgical expectations and the necessity of close monitoring postoperatively is crucial. Candidates must understand that their vision will be blurry for the first few days due to the intraoperative air bubble injected into the AC during surgery. Successful donor adhesion requires faithful supine positioning for the first 72 hours. Patients may sit up to eat, use the bathroom and bathe but are typically required to spend 45 to 50 minutes of every hour with their heads flat and chins elevated about 30 degrees. Maintaining the proper gas fill is critical for success, which is why tubes, aphakia and major iris defects are relative contraindications. It is a challenge to keep air in these eyes, which makes attachment so difficult.
Injecting sulfur hexafluoride gas in place of air into the AC, a technique used in vitrectomies, is favorable due to its extended duration—about four to seven days of gas support on the donor—compared with air, which dissipates more rapidly.7 Due to risks of pupillary block from either bubble type, one or more peripheral iridotomies (PIs) should be placed inferiorly preoperatively with a YAG laser, as the bubble will block a superior PI when the patient is upright. Some choose to create a surgical PI intraoperatively, which does not come without risks. Surgical PIs can bleed, sometimes in delayed fashion.8 If blood is present in the AC, clots and fibrin may form.
Given the fragility of the tissue, manipulating the donor graft can be the most challenging part of performing a successful EK, especially a DMEK. The graft is susceptible to expulsion from an incision wound and may invert. If the patient is pseudophakic with posterior opacification, consider delaying the YAG.
The flattening of the AC in an EK, which is key for unfolding, can cause the vitreous to prolapse through a large capsulotomy.
Patients who have undergone a vitrectomy are usually not good candidates for an EK. In some cases, the AC will not shallow when fluid is let out of the wound; the iris sags posteriorly like a hammock while the eye softens and wrinkles. This is problematic because the forward movement of the iris is needed to hold the donor tissue partially open long enough for the surgeon to put in the initial bubble.
New techniques, especially in the arena of donor preparation, have made complex, delicate and sometimes frustrating DMEK surgeries more manageable. Corneal surgeons have slowly adopted DMEKs in part because of difficulties encountered with donor preparations and concerns about the potential loss of donor tissue. Early adopters reported tissue wastage and a failure rate of one in three during donor peeling.9
Lawrence Tenkman, MD, a corneal surgeon out of Louisville, KY, has modified the Giebel 2008 SCUBA (submerged cornea using backgrounds away) technique with impressive success, reporting only one tissue failure in over 600 DMEK cases. Scored with a blunt Y-hook, the donor edge is stained with VisionBlue, lifted with a glide technique and peeled by corridors, minimizing tension. This groundbreaking method provides guidance in handling excessive separation resistance and small breaks that form during peeling that Dr. Tenkman refers to as “horseshoe tears.”10
Other improvements facilitating the rise of DMEK procedures include instrumentation, such as the Straiko Modified Jones Tube, and Eye Bank stamping of an “S” (signifying “stroma”) on the donor tissue to prevent inverted grafts (iatrogenic graft failure can occur when a surgeon mistakenly places the endothelium in contact with the stroma).11
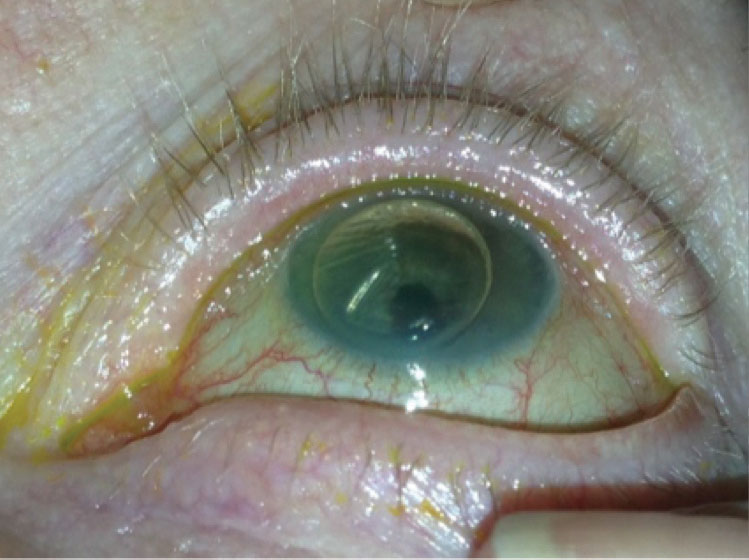 |
This is an example of the bubble that can be seen in the supine position one day after DMEK surgery. |
Post-op Management
On day one of the postoperative period, mild to moderate corneal edema with folds is expected. A bandage contact lens may have been placed in the operating room. The patient should be reassured that blurry vision is normal due to the bubble.
The goal is an attached DMEK donor and a deep AC with air filling 50% to 70% of the chamber. It is also imperative to ensure there is not an air-induced pupillary block, which pushes the pupil back against the lens and seals it to prevent the anterior flow of fluid. If the inferior PI is not functioning, the aqueous produced by the ciliary body cannot flow into the AC, and the iris bulges forward. Functionally, this is similar to acute angle-closure glaucoma and requires immediate action. Surprisingly, some patients with this issue may experience symptoms of nausea but not eye pain.
The initial and least invasive approach involves putting the patient in a full supine position, parallel to the ground, and instructing them to look in the extreme superior gaze. This technique moves the bubble inferiorly in the AC, occasionally with enough force to break the adhesion between the iris and the cornea. If a closed PI is present, attempt to reopen it via YAG laser.
Sometimes, supine positioning is not enough, and performing a paracentesis is necessary. Sterile conditions must be prioritized, preferably with betadine 5% and antibiotic drops to the ocular surface. Attached to a 1mL syringe, a 30-gauge needle should be injected at the limbus and parallel to the iris to avoid the DMEK graft. The needle should be inserted into the bubble, and the plunger should be slowly withdrawn to let enough air out so the eye is no longer firm. Sometimes, re-bubbling is necessary to ensure proper peripheral donor attachment, especially in cases of a total graft detachment. If the graft is not detached by more than a third or a fluid cleft is present, it may only require observation.
Even if postoperative findings are normal, positioning, restrictions and drop scheduling should be reemphasized to the patient. By day three or four, there should be much less corneal edema, and the air bubble should only fill about 25% of the AC. Generally, more air time is needed if there is focal edema from the DM separation that is significant enough to cause blurry vision, in which case, patient instructions should favor positions that allow the bubble to press as directly against the dehisced zone as possible. At this point, the patient may not need to adhere to the supine position.
After one week, the bandage lens can be removed, and if a single suture is present, it can be taken out. The use of topical antibiotics can be discontinued three days after the suture is removed. It is appropriate to order anterior segment OCT imaging and/or endothelial cell counts at this stage. Beginning to slowly taper the steroid is not suggested until three to four months after surgery. Some surgeons recommend complete discontinuation by six months, but most experts agree that a daily dose of a topical steroid should be used indefinitely to reduce the risk of rejection.12
The learning curve is steep, but in most cases, outcomes from a DMEK are superior to those from a DSAEK, which is largely due to the problems that arise from the stroma-to-stroma interface of DSAEK grafts.13 A tissue with a certain radius of curvature confining itself to a smaller radius of curvature results in micro-irregularities. Bare stroma cannot be avoided in these cases, leading to edema and a significantly longer healing time. Eight percent to 10% of DSAEK grafts are rejected, according to recent studies.14 DMEK grafts, on the other hand, enjoy a rate of just above 1%.14
Postoperative stability following a DSAEK may take four to six months and delay a prescription update until then. In contrast, a DMEK mimics normal anatomy and is essentially refractive-neutral, creating a negligible hyperopic shift of only about +0.25D compared with a +1.00D to +1.50D shift in a DSAEK. DMEK patients may be ready for an updated prescription in one to three months. Nearly 50% of DMEK patients achieve 20/20 six months postoperatively, and some surgeons boast a higher rate.14 A much smaller percentage of patients who have had a DSAEK achieve 20/20, and they only do so when very thin tissue is used. More often than not, patients end up with a BCVA ranging from 20/20 to 20/60.15
Unfortunately, astigmatism created by a DSAEK is posterior and not easily measurable and, therefore, limits combined cataract procedures to implant a non-toric intraocular lens (IOL). But, DMEK patients are far more predictable. Using a slightly adjusted myopic target, toric IOLs can be successfully used.16 Commonly, EKs are combined with cataract surgery for convenience and a quicker recovery with a single procedure. Phakic patients over 50 years old who have moderate guttata may even benefit from a proactive approach. We know endothelial cell loss accelerates following cataract surgery, which could turn a mild FED case into a severe case requiring an EK.
 |
| Ideal donor attachment from DMEK surgery looks like this one-week postoperatively. |
Looking Toward the Future
The future of lamellar keratoplasties is bright. Since 2006, femtosecond lasers have been used to produce custom trephination patterns. Femtosecond laser-enabled keratoplasty has been praised for achieving better donor alignment, resistance to leakage, faster healing and less postoperative astigmatism. With the emergence of femtosecond technology, improvement in descemetorhexis is being examined and may prove to be a useful adjunct in DMEK and DSAEK cases.17 Pre-DMEK procedures, which are thought to include Dua’s layer, are being seriously studied now. At 25µm to 30µm in thickness, these keratoplasties have an advantage over the ultra-thin 10µm to 15µm DMEK donors.18
Our role as optometrists goes well beyond visual rehabilitation. From surgical consultation and preoperative YAG PI to paracentesis and suture removal, optometrists across the country are learning how to do more. Education expansion and progressive legislation has allowed many to play an integral role in the perioperative care of corneal transplantation. Working together with cornea surgeons will allow for fluid comanagement and undoubtedly better outcomes for our patients, as the ultimate goal of any keratoplasty is to improve quality of life through increased vision and/or comfort.
Dr. Steele practices consultative, medical and surgical optometry at Bennett & Bloom Eye Centers in Louisville, KY. He is also an American Academy of Optometry fellow, an adjunct professor at the Indiana University and the University of Alabama-Birmingham schools of optometry, an attending for forth-year interns and ocular disease residents and a lecturer for continuing education courses. He graduated with honors from the Ohio State University College of Optometry and completed resident training in ocular disease with Bennett & Bloom.
| 1. Amouzegar A, Chauhan SK, Dana R. Alloimmunity and tolerance in corneal transplantation. J Immunol. 2016;196(10):3983-91. 2. Huang A, Bogucki J, Sheybani A. Faculty of 1000 evaluation for Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty versus penetrating keratoplasty: a randomized multicenter clinical trial. F1000Prime. December 2011. 3. Daneshgar F, Fallahtafti M. ‘Expanding bubble’ modification of ‘big-bubble’ technique for performing maximum-depth anterior lamellar keratoplasty. Eye (Lond). 2011;25(6):803-8. 4. 2016 eye banking statistical report. Eye Bank Association of America. 2017:5. 5. 2017 eye banking statistical report. Eye Bank Association of America. 2018:9. 6. Le R, Yucel N, Khattak S, et al. Current indications and surgical approaches to corneal transplants at the University of Toronto: a clinical-pathological study. Can J Ophthalmol. 2017;52(1):74-9. 7. Von Marchtaler PV, Weller JM, Kruse FE, et al. Air versus sulfur hexafluoride gas tamponade in Descemet membrane endothelial keratoplasty: a fellow eye comparison. Cornea. 2018;37(1):15-9. 8. Hlinomazová Z, Horácková M, Pirnerová L. DMEK (Descemet membrane endothelial keratoplasty)—early and late postoperative complications. Cesk Slov Oftalmol. 2011;67:75-9. 9. Lie JT, Birbal R, Ham L, et al. Donor tissue preparation for Descemet membrane endothelial keratoplasty. J Cataract Refract Surg. 2008;34(9):1578-83. 10. Tenkman LR, Price FW, Price MO. Descemet membrane endothelial keratoplasty donor preparation: navigating challenges and improving efficiency. Cornea. 2014;33(3):319-25. 11. Veldman PB, Dye PK, Holiman JD, et al. Stamping an S on DMEK donor tissue to prevent upside-down grafts: laboratory validation and detailed preparation technique description. Cornea. 2015;34(9):1175-8. 12. Price MO, Scanameo A, Feng MT, et al. Descemet’s membrane endothelial keratoplasty: risk of immunologic rejection episodes after discontinuing topical corticosteroids. Ophthalmology. 2016;123(6):1232-6. 13. Marques RE, Guerra PS, Sousa DC, et al. DMEK versus DSAEK for Fuchs’ endothelial dystrophy: a meta-analysis. Eur J Ophthalmol. April 1, 2018. [Epub ahead of print]. 14. Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(2):295-310. 15. Guerra FP, Anshu A, Price MO, et al. Descemet’s membrane endothelial keratoplasty: prospective study of one-year visual outcomes, graft survival and endothelial cell loss. Ophthalmology. 2011;118:2368-73. 16. Schoenberg ED, Price FW Jr., Miller J, et al. Refractive outcomes of Descemet membrane endothelial keratoplasty triple procedures (combined with cataract surgery). J Cataract Refract Surg. 2015;41(6):1182-9. 17. Mckee HD, Jhanji V. Femtosecond laser-assisted graft preparation for Descemet membrane endothelial keratoplasty. Cornea. 2018;37(10):1342-4. 18. Narang P. Pre-Descemet’s endothelial keratoplasty. Management of PHACO Complications: Newer Techniques. 2014:110-4. |


