 Contact lens wearers come to us with the hope of achieving improved vision without the need for glasses, and we are quite capable of satisfying this desire even in the most challenging contact lens fits.
Contact lens wearers come to us with the hope of achieving improved vision without the need for glasses, and we are quite capable of satisfying this desire even in the most challenging contact lens fits.
Oftentimes, however, some of our fitting strategies will give patients great vision under normal light condition, but can pose challenges in low-light levels, such as in the evening or in dark rooms.
In instances when we have reached the limits of what our contact lens options can achieve to ensure excellent acuity in all visual settings, we feel that it is important to discuss the role of pharmaceuticals that might be capable of helping patients further. Using every tool at our disposal may help to decrease the number of patients dropping out of lenses by eliminating decreased vision in low-light levels as a motivating factor.
Normal Physiology
In a normal individual, the pupils respond directly and consensually to increased light levels by decreasing in size. As such, when exposed to low light levels, a patient’s pupils will equally dilate as a result. As a pupil dilates, refractive aberrations of the eye that are not exposed under normal light levels become exposed. This is most evident in our post-refractive surgery patients.
Patients who have undergone radial keratotomy (RK), photorefractive keratectomy (PRK) and laser in-situ keratomileusis (LASIK) are particularly susceptible to the effects of variation in light levels on their vision. These patients may refract to 20/20 but report a significant subjective disruption to their vision under low-light levels.
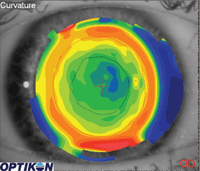
1. Topography of a patient who has had LASIK. The black line represents the shape of the pupil under bright light levels. The red line represents the shape of the pupil under dim light levels.
Under regular illumination, the pupils are smaller than the treatment zone. As such, the irregularity in the cornea created by the outer edge of the treatment zone, or by the visible incisions for those patients who have had RK, is not interfering with the vision.
For these patients, under situations of lower light levels, the pupils dilate, allowing higher-order aberrations (HOAs) created by the edge of the treatment zones to now interfere with the vision (Figure 1). Objectively, we have instrumentation that has the ability to measure these higher-order aberrations. Subjectively, we now even have access to refractive systems that can also correct for these HOAs.
Pupil Control Efforts
Although these systems can help reduce some of these challenges, they often will not completely eliminate the visual issues experienced by these patients. A strategy that works well clinically is to control the pupil size.
Pupil control can be accomplished with a number of pharmaceutical agents. For example, pilocarpine is capable of pupil control, but this pharmacological agent will also induce a myopic shift due to a contraction of the ciliary muscle. Dapiprazole, an alpha-adrenergic antagonist, can control pupil dilation, but its side effect profile, which includes burning and conjunctival hyperemia, often does not make it a practical solution.1
Brimonidine is a unique molecule that inhibits pupil dilation through a unique mechanism and has a low side effect profile relative to the other two agents discussed.

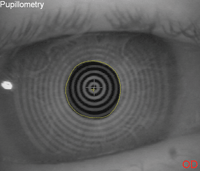
2a (left). Pupil image taken using infrared imaging before treatment with brimonidine.
2b (right). The same patient's pupil taken under the same conditions 30 minutes after instillation of 0.1% brimonidine.
How Does Brimonidine Work?
Brimonidine is an alpha-2 adrenergic agonist.2 We understand its critical role in glaucoma management. Alpha-2 receptors are located on the presynaptic nerve endings of the dilator muscle. Brimonidine binds to those receptors and, by doing so, inhibits further release of neurotransmitter into the synaptic cleft. Thus, when brimonidine is introduced to the eye, the dilator muscle will experience reduced activity, causing less pupil dilation and resulting in a more miotic pupil (Figure 2).
Where is it Found?
Brimonidine is commercially available as Alphagan. Three concentrations of brimonidine are available: 0.1%, 0.15% and 0.2%.2 A recent study showed identical results with pupil size control under low-light levels with 0.1% and 0.2% brimonidine. It is approved by the FDA to be used in a regimen of 1gt TID for IOP reduction in glaucoma patients.2
The discussion in this article is a non-FDA approved use of brimonidine, and should be discussed with patients before being considered.
For patients who would benefit from this medication, we usually recommend instilling drops in both eyes 30-60 minutes before critical viewing tasks in dim illumination.
How to Identify Candidates for Treatment
A very simple test in the exam room can be used to determine who may be a good candidate for treatment using brimonidine drops. If you suspect significant differences in visual quality under normal and dim light levels, darken the exam room and have the patient look at the distance visual acuity chart. Make sure to reduce as much light in the exam room as possible, including the monitor of any computer screens.
Next, occlude one of the patient’s eyes by holding the occluder on an angle over the non-viewing eye, so that it is unable to view the distance target but it is still possible for you to place a penlight behind the occluder. Then, simply shine a light source (such as a transilluminator or penlight) at the patient’s non-viewing, occluded eye, and have the patient report whether the vision has improved with the introduction of light to the non-viewing eye.
If the patient reports an improvement in vision, he or she may prove to be an appropriate candidate for treatment with brimonidine drops.
Contact Lens Applications
As discussed earlier, controlling the pupil dilation response under dim illumination conditions for post-refractive surgery patients provides better visual outcomes for those experiencing decreased vision under those circumstances. But there are a number of potential applications for contact lens wearers as well.
• Orthokeratology. These patients often do very well in regular light levels. However, if the pupil dilates larger than the treatment zone, patients may experience decreased visual outcomes under low-light levels. Additionally, you may rule out some patients from pursuing orthokeratology because of the limitations of larger pupils. Either of these patient profiles may benefit from brimonidine treatment.
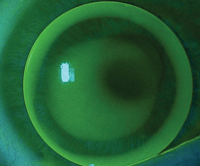
3. The right eye of a patient with keratoconus wearing a GP lens that is 9.0mm in diameter. At the slit lamp, the pupil is constricted because of the light being shone at the eye during the examination. The pupil is coming close to the edge of the lens on the superior nasal aspect of the lens.
• Gas permeable (GP) lens wearers. GPs are typically smaller than the diameter of the cornea. Most designs are significantly larger than the pupil under low-light levels and do not cause any visual issues. If a GP wearer does present with complaints of decreased vision in dim light, the first consideration should be an attempt to increase the diameter of the lens. For regular corneas, that is a relatively easy task. But for patients with corneal irregularities, that may prove to be a challenge.
It can sometimes be difficult to increase the size of the lens and also maintain an acceptable fit for patients with ectatic corneas, such as those seen in our keratoconus patients. This may leave patients dependent upon small-diameter lenses. If the lens is decentered because of centration over the cone, this can create a challenge for patients in low-light levels (Figure 3). These patients would likely benefit from brimonidine treatment.
Additionally, aspheric multifocal GP lens wearers under certain circumstances may also benefit from pupil control with brimonidine. Regardless of whether you work with multifocal GPs that have an aspheric front surface, back surface or a combination of the two, these lenses are designed with their distance optics in the center of the lens and the near optics progressing towards the periphery. As such, it is conceivable to understand a situation in which a patient with a well-centered lens would see well in the distance in regular illumination, but experience some visual disturbance under low light levels if the near aspheric zones interfere with the pupil.
Fortunately, we can manipulate the distance zone of the lens and increase its diameter, creating a larger distance zone through which the patient can view. However, this strategy comes with its own challenge. The size of the near zone of the lens decreases, potentially making it more difficult for the patient to access the near zone when needed. In this case, brimonidine for low-light levels, in particular driving in the evening, may serve these patients well.
Through a Lens, Darkly
Using brimonidine to keep your patients in their contact lenses is a novel concept; however, there are some potential downsides associated with its use. For example, some patients using brimonidine may experience side effects such as dry mouth and fatigue. It is also important to be mindful of toxicity over time when using higher concentrations of the agent. It also cannot be used with MAO inhibitors, and there are some concerns about its use in patients with elevated blood pressure.
In our quest to achieve the best possible visual outcomes and wearing experience for our contact lens patients, we need to keep non-traditional solutions in mind to meet their visual needs. Consider pupil control with brimonidine for those contact lens patients complaining of significant visual changes in dim illumination settings.
1. Canovetti A, Nardi M, Figus M, Fogagnolo P, Benelli U. Aceclidine, brimonidine tartrate, and dapiprazole: comparison of miotic effect and tolerability under different lighting conditions. J Cataract Refract Surg. 2009 Jan;35(1):42-6.
2. Shemesh G, Moisseiev E, Lazar M, Kesler A. Effect of brimonidine tartrate 0.10% ophthalmic solution on pupil diameter. J Cataract Refract Surg. 2011 Mar;37(3):486-9.


