 This year we have seen the re-release of several well-known and trusted medications in new formulations and with new indications for use. Reformulations are changes in a drug’s molecular formulary—any combination of changes in active ingredient concentrations, inactive components or changes in the means of drug delivery.1 In 2008, the FDA’s Center for Drug Evaluation and Research approved 89 new drugs, of which five were designated for ocular use.2 Four of these five drugs were either reformulations, expanded labels or new indications (repurposing).1 These statistics reflect recent observations: 50% to 75% of drug approvals are reformulations and expanded labels.1
This year we have seen the re-release of several well-known and trusted medications in new formulations and with new indications for use. Reformulations are changes in a drug’s molecular formulary—any combination of changes in active ingredient concentrations, inactive components or changes in the means of drug delivery.1 In 2008, the FDA’s Center for Drug Evaluation and Research approved 89 new drugs, of which five were designated for ocular use.2 Four of these five drugs were either reformulations, expanded labels or new indications (repurposing).1 These statistics reflect recent observations: 50% to 75% of drug approvals are reformulations and expanded labels.1
A Perfect Example
The latest reformulation to hit the market is TobraDex ST (0.3% tobramycin/0.05% dexamethasone ophthalmic suspension, Alcon), a new mixture of the old drug TobraDex (0.1% dexamethasone/0.3% tobramycin ophthalmic, Alcon), which was approved by the FDA in 1988. With over 16.3 million prescriptions written between 2001 and 2006, TobraDex is the most widely prescribed steroid/antibiotic ophthalmic combination product.3
The new formulaic adjustment for TobraDex ST decreases the amount of steroid (from 0.1% to 0.05%) and adds pharmaceutical grade xanthan gum—an inactive ingredient inserted to stabilize the combination and allow greater delivery of each drug to the eye. Xanthan gum is a water-soluble polysaccharide that is well tolerated in human eyes and increases comfort when inserted in formulations.4 The use of xanthan gum has been found to enhance contact time of the drug and thus provide improved drug delivery.5 This means that the manufacturer can use lower concentrations of the drug and still have similar concentrations delivered to tissues.3
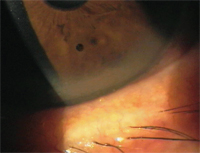
A peripheral corneal infiltrate secondary to staph blepharitis. This is a good indication for TobraDex ST.
The xanthan gum and tobramycin interaction-based suspension technology of TobraDex ST reduces settling of dexamethasone particles and improves suspension characteristics, which in turn reduces viscosity. The viscosity increases after mixing with tears; the interactions between xanthan gum and tobramycin are interrupted by the pH and ionic content of tears. This enhanced viscosity allows for longer retention of the formulation in the eye and improves the ocular bioavailability of the drug.3
TobraDex ST suspension was formulated to enhance bioavailability to targeted tissues, making it an appropriate treatment choice for acute blepharitis and other inflammatory conditions. The overall effect is an enhancement of both anti-inflammatory and antibacterial action, when compared to the original formulation.6
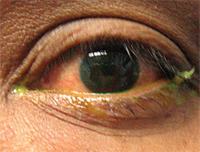
This combination drug is used for any ocular inflammation associated with a potential secondary bacterial infection. The dexamethasone treats the inflammation, while the tobramycin prevents a secondary infection.
TobraDex ST is a good treatment for acute bacterial conjunctivtitis with inflammation.
The Research
When the ocular tissues of rabbits were treated with TobraDex ST, greater concentrations of both tobramycin and dexamethasone in the tear film were observed.3 In fact, 10 minutes after dosing with TobraDex ST, there was an 8.3-fold increase in tobramycin concentration in the rabbit tear film compared with TobraDex. Concentrations of the two agents in ocular tissues from rabbits exposed to TobraDex ST were up to 12.5-fold greater than TobraDex after one hour. Additionally, xanthan gum significantly improved response to experimental Pseudomonas keratitis in rabbits.7 The suppression of inflammation with the new formulation was notably better than that achieved by the standard. Keep in mind: The new formulation contains half the dexamethasone concentration as the standard.7 The distribution of dexamethasone in the aqueous humor of humans showed that lower concentrations of dexamethasone in the TobraDex ST formulation still translated to higher concentrations in the aqueous humor. This new and improved formulation is expected to be more clinically useful.3 TobraDex ST is also now in clinical trials as a treatment for blepharitis (see, Dr. Abelson’s “Diagnose That Disease: Blepharitis” p. 10-11).8
A Valuable Addition
TobraDex ST—with the more efficient drug delivery and evidence of increased effectiveness in suppressing inflammation and killing bacteria—will be a valuable addition in multiple clinical settings and is expected to offer a useful alternative to its predecessor, TobraDex, in treating ocular conditions of the anterior segment.
1. Abelson MB. Drug development: Old drugs, new formulas. Rev Ophth. 2010 Oct;17(10):100-4.
2. U.S. Food and Drug Adminstration. CDER Drug and Biological Approvals for Calendar Year. Available at: www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/default.htm. (Accessed October 2010).
3. Scoper SV, Kabat AG, Owen GR, et al. Ocular distribution, bactericidal activity and settling characteristics of TobraDex ST ophthalmic suspension compared with TobraDex ophthalmic suspension. Adv Ther. 2008 Feb:25(2):77–88.
4. Ceulemans J, Vinckier I, Ludwig A. The use of xanthan gum in an ophthalmic liquid dosage form: rheological characterization of the interaction with mucin. J Pharm Sci 2002 Apr;91(4):1117-27.
5. Albasini M, Ludwig A. Evaluation of polysaccharides intended for ophthalmic use in ocular dosage forms. Farmaco 1995 Sep;50(9):633-42.
6. U.S. Food and Drug Administration. CDER NDA approval for Tobradex ST. Available at: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/index.cfm?fuseaction=Search.DrugDetails. (Accessed 2010).
7. McCormick C, Caballero A, Tang A, et al. Effectiveness of a new tobramycin (0.3%) and dexamethasone (0.05%) formulation in the treatment of experimental Pseudomonas keratitis. Cur Med Res Opin. 2008 Jun;24(6):1569-75.
8. Alcon Research. A study to evaluate the clinical efficacy and safety of tobradex st compared to AzaSite in the treatment of subjects with moderate to severe chronic blepharitis. 2010 Apr. Available at: www.clinicaltrials.gov/ct2/results?term=tobradex+st. (Accessed October 2010).


