 |
Although it’s estimated that roughly 20% of patients with herpes simplex virus (HSV) keratitis will develop herpes stromal keratitis (HSK), I’d estimate 50% of HSV patients referred to our cornea clinic from ODs have HSK.1 This suggests we are more comfortable identifying and treating dendritic keratitis than we are with HSK. And it’s not surprising, given just how repeatable the presentation of the dendrite is, compared with the extremely variable nature of HSK. Let’s take a look at HSK and discuss the diagnostic clues different cases offer us.
HSK Pathology
HSK is a widely studied yet poorly understood pathology. Viral replication within the stroma probably isn’t taking place, and viral proteins haven’t been isolated in eyes with experimental HSK.2This intuitively makes sense when we consider viral pathophysiology: viruses are obligate intracellular pathogens, and the sparsely distributed keratocytes would present a poor target for infection, as cell-to-cell propagation would be limited by distance and lack of accessible blood or lymph channels. Rather than actual viral infection, more likely mechanisms of HSK inflammation are either an immune response to shed viral antigens or perhaps a form of autoimmunity to corneal autoantigens “turned on” by the original HSV infection.2 Regardless of the precise mechanism, the subsequent immune response—while involving multiple classes of immune cells—is primarily mediated by T-lymphocytes.
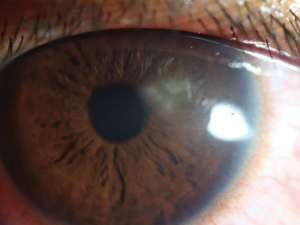 |
| Fig. 1. Typical sub-dendritic HSK lesions, as seen here, sometimes occur following dendritic keratitis. |
Clinically, for all HSV keratitides, a positive previous HSV history is strongly suggestive that any active stromal keratitis is linked to the virus. However, lack of a known HSV history doesn’t eliminate the possibility of HSK, as it may occur spontaneously without identifiable risk factors or serendipitously in an eye with risk factors for more acutely severe corneal diseases such as microbial keratitis. If the clinical picture doesn’t support a microbial form, consider HSV.
The most common, easiest to identify type of HSK is the sub-dendritic form. Its timing may immediately follow the active dendrite or occur months later, but as it is immediately below a dendrite or dendriform scar, it’s not challenging to diagnose. Its active form has zones of irregular corneal inflammation and migrating white blood cells that will give the anterior cornea a grainy or powdery appearance. Though this form of HSK is relatively easy to diagnose, many other types are not so clear cut (Figure 1).
Case Report
A previously healthy 24-year-old with no interesting ocular history developed the pathology that led to an atypical lesion three weeks prior to being seen (Figure 2). The infiltrate is particularly deep, involves the paracentral cornea and has density consistent with microbial keratitis. While its shape is somewhat irregular for a microbial source (which tends to be round or oval), they can’t be ruled out. A closer look at the clinical presentation and history will help reveal the true pathology, however.
First, the patient has no risk factors or history. Risk factors, such as contact lens use, trauma and severe ocular surface disease, are practically required for HSK, so while a microbial source is not impossible, it is unlikely.
Next, the vascular leash on the infiltrate is a classic sign of HSV keratitis. Though not all HSK cases vascularize, it is the most common connecting diagnosis between non-peripheral keratitis and vascularization. Any active, non-limbal keratitis with vascularization should suggest HSV, and any anterior scar with vascularization should point to previous HSV as a possible cause.
Corneal neovascularization (CN) is not unique to HSV keratitis, but HSV keratitis is the most common cause of CN in the United States.3 Although the specific source of CN in HSV is vague, all inflammatory cells involved in HSK directly upregulate, or produce cytokines that upregulate VEGF isoforms. In mouse models, mice without a thymus and thus without T-cells don’t develop HSK or subsequent CN, suggesting a primary role of T-cells and their derivatives in these processes.4 Keeping all this in mind, when you see an isolated infiltrate with vascularization that isn’t at the limbus, HSV is the chief differential.
However, an infiltrate such as this, given its location and the associated risk for vision loss, should prompt cautious management. Given this ulcer’s appearance and location, clinicians should consider a superinfection with microbial etiology over an HSV lesion. In this case, the eye was cultured and placed on topical antibiotic for coverage as well as the more therapeutic oral antiviral. When cultures returned negative, corticosteroid was added, antibiotic was discontinued and the patient responded well.
 |
| Fig. 2. Although this atypical infiltrate suggests microbial keratitis, a closer look reveals HSK. |
Treatment
For HSK, antiviral and anti-inflammatory therapy is conventional, and according to the Herpetic Eye Disease study, long-term use of acyclovir for stromal keratitis is helpful in reducing recurrence.5 Unfortunately, treatment failure occurs in a percentage of patients due to either worsening HSK or HSK-related CN and its sequela, such as lipid exudate and corneal opacification. Luckily, other medical options can help.
Research shows low concentration cyclosporine—an inhibitor of T-cells, which seem to be the primary force behind HSK—can be effective in non-responsive HSK in both animal studies and clinical case series.6,7 Further, we may be able to indirectly reduce development of HSK-associated CN using cyclosporine. Treating HSK is one of the few non-dry-eye medical applications for Restasis (cyclosporine ophthalmic emulsion, Allergan), as its 0.05% concentration seems to be sufficiently high, compared with other medical applications of topical cyclosporine that require compounded drops at 0.5% to 2%.6,7 However, clinicians should be cautious when using Restasis here, as it’s specifically cautioned against in the package insert.8 Patients should be appropriately counseled and maintained on a suppression dose of oral antiviral throughout treatment.
A newer treatment option, Xiidra (lifitegrast, Shire Pharmaceuticals), is involved in downregulating T-cell activity and may prove its worth over time as well. However, no studies yet show its effect for HSK.
Matrix metalloproteinases (MMPs) assist in the development of CN by degrading corneal connective tissue and allowing space for formation of blood vessels. In HSK cases with early CN, a two-to-three month course of doxycycline to reduce MMP is a reasonable, noninvasive option to attempt to reduce development of CN. As with cyclosporine, this application makes the most sense as a preventative therapy rather than a treatment of existing CN.
Finally, when large vessels are established but the process is still relatively early, the CN may be susceptible to intrastromal or subconjunctival dosing of Avastin (bevacizumab, Genentech), which we used to terrific effect with our patient. This approach works best early in the process because chronically established CN is less susceptible to complete resolution with anti-VEGF therapy. As with Restasis, this would be considered an off-label use of the medication and requires appropriate patient consent.
HSK can be quite difficult to treat. Compared with dendritic keratitis, HSK treatments are longer and more likely to lead to permanently compromised vision. Additionally, the lesions can masquerade as any number of stromal keratitides, making diagnosis challenging. But keeping HSV near the top of your differential for any unusual unilateral keratitis can help you prepare for the possibility of dealing with a viral etiology and initiate appropriate treatment in a timely manner.
1. Sheppard JD, Wertheimer ML, Scoper SV. Modalities to decrease herpes simplex seratitis reactivation rates. Arch Ophthalmol. 2009;127:852-6.
2. Dana MR, Qian Y, Hamrah P. Twenty-five year panorama of corneal immunology. Cornea. 2000;19:625-43.
3. Schwartz GS, Harrison AR, Holland EJ. Etiology of immune stromal (interstitial) keratitis. Cornea. 1998;17(3):278-81.
4. Gimenez F, Suryawanshi A, Rouse BT. Pathogenesis of herpes stromal keratitis-a focus on corneal neovascularization. Prog Rein Eye Res. 2013;33:1-9.
5.The Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex viral eye disease: Effect on prevention of epithelial keratitis and stromal keratitis. Arch Ophthalmol. 2000;1030-6.
6. Yoon KC, Heo H, Kang IS, et al. Effect of cyclosporin A on herpetic stromal keratitis in a mouse model. Cornea. 2008;27:454-60.
7. Roa SN. Treatment of herpes simplex virus stromal keratitis unresponsive to topical prednisolone 1% with topical cyclopsorine 0.05%. Amer J Ophthalmol. 2006;141:771-2.
8. Allergan. Restasis package insert. Irvine, CA, 2013.


