Dry eye is responsible for significant drop out rates among contact lens wearers, and in turn, accounts for a potentially significant income loss to contact lens practices. In a 2010 survey, the mean dropout rates among contact lens wearers was reported at 15.9% in the United States and 17% in the Americas (including the United States).1 This represents a loss of about one in six contact lens wearers and a significant revenue loss to the practice—calculated at approximately $21,695 over the lifetime of a single contact lens dropout.1 With reports suggesting that over half of our contact lens wearing population complains of dryness and discomfort, how do we resolve these issues and ensure comfortable lens wear for our patients?
Breaking Down Rewetting Drops
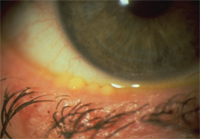
1. Meibomian gland dysfunction showing full gland orifices.
Like human tears, contact lens care products include electrolytes such as sodium chloride, potassium chloride, calcium chloride, magnesium chloride and magnesium phosphate. Chemically, electrolytes are substances that disassociate into ions in solution and acquire the capacity to conduct electricity. The electrolyte composition of tears is similar to that of blood plasma. Electrolytes also provide some buffering against changes in pH, and their concentrations directly affect the product’s tonicity.2
Rewetting drops also contain buffers. Buffers are created by balancing concentrations of weak acids and conjugate bases (e.g., boric acid and sodium borate) to create a stable balance that resists changes in the solution’s pH. Buffers maintain the pH within a defined range so as to control chemical reactions and prevent product degradation. Phosphates, borates and citrates are common chemical buffers with both common and unique properties. Phosphate buffers are the most physiologic of common buffers; they are non-toxic to cells and mimic certain components of extracellular fluids. Borates can buffer and also act as surfactants (detergents) providing slipperiness to solutions. Borates act as sequestering agents and readily bind with calcium. They provide an added antimicrobial kick against bacteria and fungi, but are generally considered to have greater cytotoxicity than phosphates. Citrates buffer against pH changes and also have chelating properties that can inhibit or remove potential protein deposits from lenses. Tromethamine is a weakly basic protein substrate used as an alkalizing agent and as a buffer. It is a proton acceptor, which combines with hydrogen ions to form bicarbonate and a buffer.
Surfactants—an English term for amphiphilic surface-active agents—are another key component in rewetting drops. These components contain both hydrophobic tails and hydrophilic heads, which provide the ability to reduce the interfacial tension between oil and water, or water and proteins. This property provides the slipperiness of detergents. Tyloxapol is a nonionic liquid polymer used as a surfactant to aid liquefaction and removal of mucous. Polyethylene glycol-11 (PEG-11) lauryl ether carboxylic.3 The Tetronic (BASF) family of surfactants is made up of tetra-functional block copolymers based on ethylene oxide and propylene oxide.4 Tetronic 1304 is the ethylene oxide/propylene oxide block copolymer used by Alcon in many of its products; a product synonym is poloxamine.
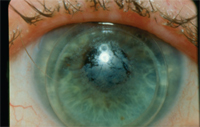
2. Staph blepharitis with filmy coating of GP lens.
Tonicity is another important part of rewetting drops. It is well recognized that simply wearing a contact lens has an impact on the ocular surface, including reduced corneal sensitivity, altered tear meniscus, increased tear evaporation and altered blink frequency. It also affects the chemical composition of human tears. More than 100 different proteins have been identified along with lipids, electrolytes, metabolites and other substances.2 Eye drops are generally made isotonic to mimic the tear film that is approximately equivalent to 0.9% NaCl and at an average osmolarity of 318 mOsm/kg.5 Hyperosmolarity is associated with all forms of dry eye disease; therefore isotonic or hypotonic solutions are preferred when treating dry eye states.
The Role of Ophthalmic Demulcents
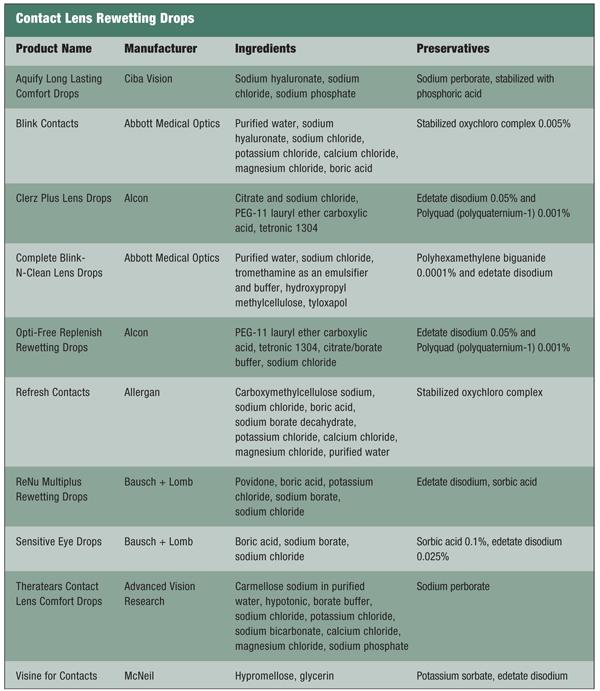
A demulcent is an agent that soothes irritation by forming a film over an epithelial surface or mucous membrane. In fact, the term demulcent is frequently used interchangeably with lubricant. The U.S. FDA regulates types and concentrations of ocular demulcents in over-the-counter formulations. Demulcents used in contact lens rewetting drops are listed in figure 3 and discussed below.
• Hyaluronic acid (HA) or its salt sodium hyaluronate: A naturally occurring disaccharide biopolymer found in the human body. Hyaluronic acid is found in human synovial fluid of knee joints, the vitreous humor of the eye, cartilage, blood vessels, extracellular matrix and skin. HA is the key water-retaining substance in human skin. Loss of HA and other polysaccharides results in thinning and increased sensitivity of skin. HA is a structural polysaccharide composed of repeating units of N-acetylglucosamine and glucuronic acid. Sodium hyaluronate solutions strongly adhere to the mucin layer of the tear film, creating a persistent water-retaining layer that resists evaporation. HA is also viscoadaptive, which means that HA rapidly thins during blinking and thickens between blinks because of a property called shear-thinning.6,7 As a result, HA containing drops tend to be long lasting. HA has considerable viscoelastic properties and a extremely high capacity to imbibe water. HA is also incorporated into some soft lenses to maintain lubrication and inhibit deposit formation. HA is found at 0.10% in Aquify Long Lasting Comfort Drops (Ciba), and at 0.015% in blink Contacts (AMO).8
• Carboxymethyl cellulose (CMC): A water soluble, high molecular weight demulcent with strong water binding and bio-adhesive qualities and is a common muco-protectant in artificial tears.6 It provides lubrication and viscosity control. The functional properties of CMC depend on the degree of hydroxyl group substitution of the cellulose structure as well as its chain length. It is found in Refresh Contacts (Allergan) at 0.5%. It is known as carmellose and is found in Theratears Contact Lens Comfort drops (AVR).
• Hydroxypropyl methylcellulose (HPMC): Also known as hypromellose, this component has a high viscosity demulcent with extended retention time. HPMC does not penetrate into the lens matrix; rather it provides a coating between the lens and the ocular surface. HPMC increases retention time on the ocular surface by absorbing the water that is present and thus delaying its evaporation. HPMC is found in Complete Blink-N-Clean Lens Drops (Abbott Medical Optics), and in Visine for Contacts (McNeil).
• Povidone aka polyvinylpyrrolidone: This component is available in artificial tears and rewetting drops in concentrations ranging from 0.1% to 2.0%. Povidone readily complexes with other wetting agents to provide coatings with long surface retention times. It absorbs up to 40% of its weight in water. Povidone is found in Renu Multiplus Rewetting Drops (Bausch + Lomb).
• Glycerin: is a humectant with high solubility in water. It is a polyol compound with applications as humectant, solvent, sweetener, and preservative in the food science industry. Glycerin is generally used in 0.2% or 0.3% concentrations combined with other lubricants for ophthalmic use. It is combined with HPMC in Visine for Contacts (McNeil).

Preservatives are Vital
Preservatives are important components in any multi-use ophthalmic preparation. Preservatives act to prevent product degradation as well as inhibit microbial contamination. Mild chemicals and/or dissipating preservatives are generally used for lens rewetters. Sorbic acid (potassium sorbate) is a natural, organic food and ophthalmic preservative with low toxicity. It is a relatively weak static inhibitor of many fungi and bacteria. Since rewetting drops are intended for use over contact lenses, harsh preservatives like benzalkonium chloride (BAK) are not allowed.
More contemporary preservatives break down when exposed to light or tears, thus reducing their potential toxicity. Theses are known as dissipating preservatives, which include sodium perborate and stabilized oxychloro complex (SOC). Sodium perborate undergoes hydrolysis in contact with water, producing hydrogen peroxide, oxygen and borate. Stabilized oxychloro complex is also known under the trade names of Purite and OcuPure. Chemically, SOC is a mixture of chlorine dioxide, chlorite and chlorate. It dissociates into oxygen, water and chlorine free radicals when exposed to light.
Polyquaternium-1 and polyhexamethylene biguanide (PHMB) are common strong disinfectant/preservatives found in contact lens multipurpose solutions. The chelating agent and antioxidant edetate disodium (EDTA) is also found in most contact lens care products and ophthalmic pharmaceuticals. EDTA boosts the effect of other disinfectants and slows deposit formation on contact lenses.
All preservatives are toxic to the ocular surface if present in sufficient concentrations. Therefore the minimal preservative concentration to balance antimicrobial efficacy and homeostatic maintenance of ocular surface structures and function is an industry challenge.
Using Rewetting Drops
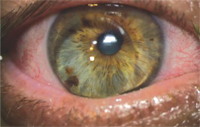
4. Tight, low-riding GP lens with general injection.
Lubricating/rewetting drops may help to relieve dry eye symptoms, or they can exacerbate the problem, depending on the dosage and types of drops chosen. What’s more, artificial tears (AT) and contact lens rewetting agents are not the same.
Rewetting agents are surface-active chemical substances that increase the spreading and penetrating properties of a liquid by lowering its surface tension. Most rewetting drops are preserved, multi-dose products that contain electrolytes, buffers and wetting agents (surfactants, demulcents and/or hyaluronic acid). Surfactants provide the slipperiness of detergents. Too much surfactant can emulsify the tear lipid layer, leading to increased tear evaporation and exacerbating dryness symptoms. Many ATs are preserved with benzalkonium chloride (BAK), which can bind to lens surfaces and become toxic to the cornea—exacerbating dryness symptoms.11,12
The chemistry of artificial tears, ocular lubricants and contact lens rewetting drops is complex. As our understanding of the human ocular surface and dry eye disease has evolved, so have tear substitutes and lens rewetting drops. Today’s products more accurately mimic human tears, provide increased tear-retention time and enhance comfort for our patients. However, patients tend not to differentiate between artificial tears and contact lens rewetting drops. It is our responsibility to educate patients in appropriate product usage. Proper and timely use of lens rewetting drops will benefit our patients and our practices.
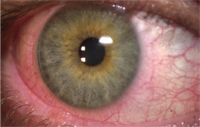
5. A reaction to soft lens MPS.
Michael Ward is the director of the Emory Contact Lens Service and an instructor in Ophthalmology at Emory University School of Medicine. He is an Honored Fellow of the Contact Lens Society of America, and a fellow of the American Academy of Optometry.
1. Rumpakis J. New Data on Contact Lens Dropouts: An International Perspective. Rev Optom. 2010 Jan.
2. Benjamin W, Hill R. Human Tears: Osmotic Characteristics. Invest Ophtlamol Vis Sci. 1983 Dec;24(12):1624-6.
3. Milne G. Gardner’s Commercially Important Chemicals: Synonyms, Trade Names, and Properties. Wiley Interscience: 2005 Jul:585.
4. BASF. Tetronic. Available at: www.worldaccount.basf.com/wa/NAFTA~en_US/Catalog/ChemicalsNAFTA/pi/BASF/Brand/tetronic. (Accessed November 2010).
5. Zhou L, Beuerman RW, Foo Y, et al. Characterisation of Human Tear Proteins Using High-resolution Mass Spectrometry. Ann Acad Med Singapore. 2006 Jun;35(6):400-7.
6. Abelson M, Anderson R. Demystifying Dumulcents. Rev Opthal. 2006 Nov:13(11):122-6.
7. Ward M. New rewetting drops improve patient comfort. CL Spectrum. 2004 Nov. Available at: www.clspectrum.com/article.aspx?article=12697. (Accessed November 2010).
8. Szczotka-Flynn L. Chemical properties of contact lens rewetters. CL Spectrum. 2006 Apr. Available at: www.clspectrum.com/article.aspx?article=104176. (Accessed November 2010).
9. Asbell P, Lemp M. Dry eye disease: the clinician’s guide to diagnosis and treatment. Thieme. 2006.
10. Carnt N, Willcox D, Evans V, et al. Corneal Staining: The IER Matrix Study. CL Spectrum. 2007 Sep.
11. Ward M. Choose Rewetting drops and Artificial Tears with Care. CL Spectrum. 2009 Jul.
12. Ward M. Contact Lens Rewetting Drops. Refractive Eyecare. 2009:11(16).


