What is a biofilm? How does it form? Is it avoidable? And, what consequences does it have regarding contact lens care education for our patients? These questions raced through my mind the first time I heard the term “biofilm.” With a thorough understanding of biofilm formation, we can better counsel patients in the proper techniques and schedules for case cleaning and replacement.
All About Biofilms
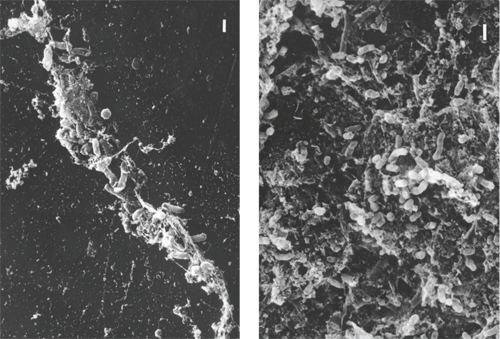
1, 2. Note the rods embedded in
the polymer matrix of the contact lens (left). A thick biofilm of
rod-shaped bacteria covers the surface of a lens case (right).
A biofilm is a group or collection of free floating microorganisms called planktonic cells that secretes its own protein-polysaccharide complex, creating an “outer shell,” or glycocalyx. I like to think of this outer covering as the new Dallas Stadium, with all the seats inside as individual organisms. The outer shell protects pathogens on the inside while continuing to recruit free-floating microorganisms to add layers to the dome. The cycle continues as the biofilm matrix becomes larger, making this complex network of microorganisms more difficult to kill.1
Planktonic cells are able to adhere to medical device surfaces, such as the polymer of the contact lens material and the contact lens case. In one study, 82% of the contact lens cases examined tested positive for pathogen contamination.2 We also know that the growth of biofilm formation occurs rapidly over the initial two hours for S. marcescens and six hours for P. aeruginosa.3 These become the anchor cells, making it easy for other cells to adhere. At this “infant” stage, the biofilm is easy to eradicate because of its loosely connected network of cells. But, once other cells build upon the anchor cells, adherence between them and the contact lens case or lens surface becomes stronger. The mature biofilm begins to produce a substance that has new properties, making it more antimicrobial resistant than individual planktonic cells.4 Subjects of one study who failed to fully air dry their case more than doubled their risk of microbial keratitis.5 Studies have found a strong correlation between the strain of pathogen in the eye infection and the contact lens case pathogen.6 Biofilms can flourish within a contact lens case—especially in that of a noncompliant lens wearer (figures 1 and 2).
Case Technology
Are all cases created equal? To answer that question, we would need to first ask: “Are all contact lens solutions created equal?” The answer to both: “No.” As we all know, there are different formulations of contact lens solutions and different contact lens case polymer formulations.
Currently, there are three different polymer plastics (polypropylene, styrene and polyethylene) that can be employed when casting a lens case. These polymers differ in rigidity and molecular weight. Additives are usually mixed in to soften the polymers and give them their shape. For example, stearic acid softens the case, but if the concentration becomes too high, it will bloom out onto the surface and react with the preservative in the solution.7 When it reacts with the preservative, it decreases the efficacy of the solution. Contact lens cases distributed and coined as “flatpaks” may be made by a plastic polymer plant in a very cost-efficient manner, but the concentration of stearic acid in these cases is unknown. Cases typically made by a major contact lens solution manufacturer are tested for safety and efficacy with their specific lens care products, contact lens materials or additional lens cleaners. So, instruct patients to use the case the solution manufacturer provides with its company name or solution name stamped on the case itself.
4. Activity of solutions when
tested against biofilm-laden lotrafilcon A lenses. Note the decrease in
disinfection efficacy of PHMB based solutions.
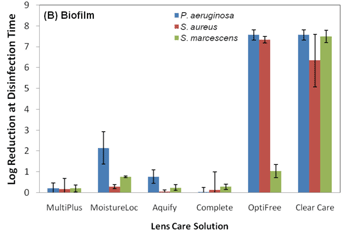
With that said, the current ISO 14729 standards for evaluating the efficacy of a contact lens solution do not require testing with a contact lens case or any contact lens material.8 These ISO standards require testing against only five planktonic organisms in a test tube environment. These organisms are Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcescens, Candida albicans and Fusarium solani.9 The current five organism strains used in ISO 14729 testing are cultured in a lab and are considered less virulent than today’s real-world mutated organisms (i.e., clinical isolates).10 Acanthamoeba is not part of the ISO testing; therefore, it is not required for solution manufacturers to challenge the efficacy of their solution against this organism. Acanthamoeba exists in two forms: an active trophozoite form that ravenously consumes bacteria and a “survival mode” cyst state that is very difficult to eradicate because of its double-layer cell wall.11 It is important to mention that all contact lens solutions pass current FDA testing criteria against the five standard ISO strains of planktonic cells, but once these solutions are tested against more virulent biofilm strains, the efficacy of the solution can change. Polyquaternium-based disinfection solutions are more effective against both standard and real-world clinical isolates strains of Fusarium solani than PHMB-based solutions.10 And, it was also reported that polyquad/aldox and hydrogen peroxide-based systems maintained their efficacy against P. aeruginosa and S. aureus biofilm-coated lotrafilon A lenses, while the efficacy of PHMB-based solutions decreased (figures 3 and 4).3 This is important because S. aureus and P. aeruginosa are some of the most cultured organisms from eye infections.12
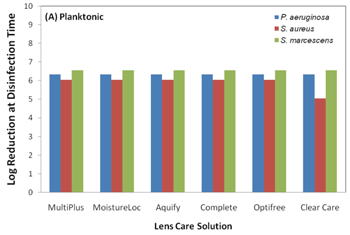
3. Disinfection activity of contact lens solutions against planktonic bacteria.
Recently, a manufacturer incorporated a silver nitrate lining in their contact lens cases in an attempt to decrease or retard biofilm formation. Here’s how this works: silver nitrate is slowly activated during the soaking cycle of a contact lens solution and is ultimately dissolved by sterile aqueous solution. The disinfection process can take up to 24 hours for silver nitrate to completely become efficacious. Thus, PHMB or biguanide is still used to increase the disinfection properties of the solution and decrease potential adverse events. Compliance with this care system differs from conventional lens case compliance because of the silver-lined case. The manufacturer recommends emptying the case of old solution, rinsing it with new solution and placing the caps tightly back on the case, so the case will not fully air dry after contact lenses have been removed from the case. Capping the lens case tightly allows the silver ions to react with the fresh lens solution residue, which continues to kill pathogens. This method reduced case contamination by 40% in one study.13
But, silver nitrate lining has a few drawbacks. Any patient with silver or metal allergies cannot use these cases as a part of their care system. Silver nitrate is a bactericidal agent only; it is not effective against Acanthomoeba.14 In fact, one study demonstrated that when the silver-lined case and solution was overwhelmed with bacteria, it had marked P. aeruginosa biocidal activity, but had minimal effects against S. aureus.15 A hydrogen peroxide-based solution is another alternative in contact lens case technologies. These systems require a catalase for neutralization of the solution, which allows it to be non-toxic to the ocular surface. Two-step hydrogen peroxide disinfection systems are the most efficacious at killing bacteria, fungi and Acanthomoeba, because the peroxide soaks at a certain concentration (usually 3%) for four hours, killing most organisms. One such study demonstrated that a four-hour 3% hydrogen peroxide soak received the best marks for preventing biofilm formation.16 But, two-step peroxide systems are very cumbersome to employ, and most of our patients do not want to comply with a two-step system in their contact lens care regimen. This is probably why most peroxide systems today are one-step systems that begin to neutralize after 15 minutes and are almost completely neutralized to sterile water 90 minutes after exposure to the catalase.17 But, the quick neutralization of a one-step system does not allow enough contact time between hydrogen peroxide and Acanthamoeba cysts to effectively eradicate them.18 This is especially important for patients who use peroxide systems in the United Kingdom, as the incidence of Acanthamoeba keratitis has been reported to be 15 times higher in the United Kingdom than it is in the United States.19
Case Replacement and Intervention
Tap water is filled with microorganisms, which can increase the likelihood of Acanthamoeba keratitis. But, in 2007, most of one study’s cohort—52%—used tap water to clean their cases, and only 20% of subjects replaced their case at least annually.20 The following year, another study found that only 3% of South Florida municipal water sources were contaminated with Acanthamoeba, but of all actual Acanthamoeba keratitis cases studied in Florida, 25% had evidence of Acathamoeba in the water supply.21
Remember, Acanthamoeba feeds off of bacteria, which is why keeping a clean, bacteria-free case should potentially limit Acanthamoeba growth.
Caution patients never to let tap water come into contact with their lenses, lens case or solution. With our consistent education efforts, the percentage of those who replace their case should increase substantially. Also, patients can interrupt biofilm formation by disinfecting their cases regularly—microwave the lens case for three minutes with contact lens solution in the case, which has been shown to completely kill all pathogens while not affecting the case shape.22 Microwave disinfection is performed by filling the wells of the contact lens case half full with contact lens solution and leaving the lid caps placed on the receptacle but not screwed on to avoid overpressure. Or, another method: Have patients empty out the case, then completely fill it with fresh new multipurpose solution during the day (without lenses) for storage. This creates a 24-hour continuous disinfection process and potentially prevents any early biofilm formation. Don’t forget, no matter how much your patients disinfect their cases, make sure they replace their lens case every three to six months per FDA recommendations.23
Patient Education
When established contact lens patients call to schedule an appointment for an exam and fitting, have them bring in all their lens cases from their house. You might be surprised how patients hoard their cases, and how many different contact lens case designs are available!
Tell your patients to throw away all cases that do not match the solution they’re currently using. Just as we are educating compliance with contact lens replacement schedules and contact lens solutions, now is the time to take action and educate our patients on biofilms and the importance of good contact lens case compliance.
The Value of Your Recommendation
We, as a profession, must still take great strides to prevent biofilm formation in contact lens cases. Contact lens solutions should be tested with lens cases and lens materials, including silicone hydrogels. Newer, more virulent clinical isolates should be challenged when testing contact lens solutions and Acanthamoeba should be considered in new disinfection standards.
But as of now, we know that some contact lens solutions have shown better efficacy against biofilms. So, remind your patients that combining different medical devices, such as cases, lenses and solutions, can be a very complex undertaking, and even though they seem extremely safe most of the time, many risks still exist. Finally, when you are prescribing the correct contact lens solution, lens material and lens case, write a prescription for each medical device employed. After all, isn’t the case and solution selection just as complex and important as a topical or oral drug?
Dr. Mayers is in private practice in Powell, Ohio. He is the founder of Mayers Eye Solutions.
1. Zegans ME, Shanks RM, O’Toole GA. Bacterial biofilms and ocular infections. Ocul Surf. 2005 Apr;3(2):73-80.
2. Gray TB, Cursons RT, Sherwan JF, Rose PR. Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br J Ophthalmol.1995 Jun;79(6):601-5.
3. Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009 Sep;28(8):918-26.
4. Farber BF, Hsieh HC, Donnenfeld ED, et al. A novel antibiofilm technology for contact lens solutions. Ophthalmology. 1995 May;102(5):831-6.
5. Edwards K, Keay L, Naduvilath T, et al. Risk factors for contact lens related microbial keratitis in Australia. Invest Ophthalmol Vis Sci. 2005;46: E-Abstract 92. Available at: http://abstracts.iovs.org/cgi/content/abstract/46/5/926. (Accessed March 2010).
6. McLaughlin-Borlace L, Stapleton F, Matheson M, et al. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998 May;84(5):827-38.
7. Personal Communication. Ralph Stone. November 13, 2009. American Academy of Optometry Meeting. Orlando, Fla.
8. Saviola JF. Contact lens safety and the FDA: 1976 to the present. Eye Contact Lens. 2007 Nov;33(6 Pt 2):404-9.
9. ISO 14729. Ophthalmic optics – contact lens care products – microbiological requirements and test methods for products and regimens for hygienic management of contact lenses. 2001. Available at: www.iso.org/iso/catalogue_detail.htm?csnumber=25382. (Accessed March 2010).
10. Hume EB, Flanagan J, Masoudi S, et al. Soft contact lens disinfection solution efficacy: clinical Fusarium isolates vs. ATCC 36031. Optom Vis Sci. 2009 May;86(5):415-9.
11. Johnston SP, Sriram R, Qvarnstrom Y, Roy S, et al. Resistance of Acanthamoeba cysts to disinfection in multiple contact lens solutions. J Clin Microbiol. 2009 Jul;47(7):2040-5.
12. Sherwal BL, Verma AK. Epidemiology of ocular infection due to bacteria and fungus. Available at: www.jkscience.org/archive/volume103/original/ocular%20infection.pdf. (Accessed March 2010).
13. Amos C. Clinical testing of the MicroBlock antimicrobial lens case. Optician. 2005;230(6008):16-20.
14. Weisbarth R, Gabriel MM, George M, et al. Creating antimicrobial surfaces and materials for contact lenses and lens cases. Eye Contact Lens. 2007 Nov;33(6 Pt 2):426-9.
15. Vermeltfoort PBJ, Hooymans JMM, Busscher HJ, et al. Bacterial transmission from lens storage cases to contact lenses—effects of lens care solutions and silver impregnation of cases. J Biomed Mater Res B Appl Biomater. 2008 Oct;87(1):237-43.
16. Wilson LA, Sawant AD, Ahearn DG. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol. 1991 Aug;109(8):1155-7.
17. Ngo W, Heynen M, Joyce E, Jones L. Impact of protein and lipid on neutralization times of hydrogen peroxide care regimens. Eye Contact Lens. 2009 Nov;35(6):282-6.
18. Hughes R, Kilvington S. Comparison of hydrogen peroxide contact lens disinfection systems and solutions against Acanthamoeba polyphaga. Antimicrob Agents Chemother. 2001 Jul;45(7):2038-43.
19. Kilvington S, Gray T, Dart J, Morlet N, et al. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci. 2004 Jan;45(1):165-9.
20. Morgan, PB. The Science of Compliance. Eurolens Research. The University of Machester.
21. Shoff ME, Rogerson A, Kessler K, Schatz S, Seal DV. Prevalence of Acanthamoeba and other naked amoebae in South Florida domestic water. J Water Health. 2008 Mar;6(1):99-104.
22. Hiti K, Walochnik J, Faschinger C, et al. Microwave treatment of contact lens cases contaminated with Acanthamoeba. Cornea. 2001 Jul;20(5):467-70.
23. FDA. Focusing on contact lens safety. Available at: www.fda.gov/forconsumers/consumerupdates/ucm048893.htm. (Accessed March 2010).


