Eye care practitioners see a variety of anterior segment diseases and disorders with similar presentations. In cases of red eyes, clinicians must determine whether the inflammatory event is sterile or infectious in nature, and then determine the appropriate course of treatment. In this article, we will review corneal inflammatory events and considerations for clinical care.
What is Inflammation?
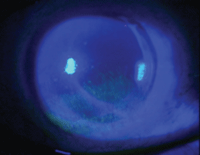
1. Superficial punctate keratitis.
Inflammation stems from the body’s active or reactive methods of protecting tissues and organs. It is an efficient, non-specific response caused by injury, infection, autoimmune processes or idiopathic conditions. During this process, the body’s white blood cells and other immune factors protect against foreign pathogens such as bacteria and viruses. In certain conditions the body becomes symptomatic, subjecting the patient to redness, warmth, swelling and pain.
Episodes can present as acute (hours to days) or chronic (weeks to years) with varying severities. Our goal is to aggressively treat acute inflammatory events and prevent chronic inflammation from damaging the ocular tissues, possibly leading to blindness.
Pathophysiology
The inflammatory response differs in vascular and avascular tissue.
In vascular tissues, inflammation is an asset that helps defend the body. Vasodilation leads to redness and an increase in size of the blood vessels. There is an increase in vascular permeability, which allows fluid to get into the affected area. Thereafter, white blood cells migrate into the tissue to fulfill their role and attempt to remove the offending agent.
 In avascular tissues, inflammation is a liability that can lead to tissue damage and scarring. The normal cornea is transparent and maintains itself as an immune privileged site, in part because it is avascular. For the cornea, the response of the limbal vasculature accounts for vessel penetration and infiltrative events.1 If not adequately treated, chronic inflammation can lead to fibrinization secondary to inflammatory cells, granulomatous formation, deposition of fibroblasts, tissue hardening and destruction, neovascularization and pannus.2
In avascular tissues, inflammation is a liability that can lead to tissue damage and scarring. The normal cornea is transparent and maintains itself as an immune privileged site, in part because it is avascular. For the cornea, the response of the limbal vasculature accounts for vessel penetration and infiltrative events.1 If not adequately treated, chronic inflammation can lead to fibrinization secondary to inflammatory cells, granulomatous formation, deposition of fibroblasts, tissue hardening and destruction, neovascularization and pannus.2
In its natural state, the cornea is 78% water. Any significant increase in water content can lead to corneal edema and a loss of transparency.3 The cornea is surrounded by fluid on both sides—tear film anteriorly and aqueous posteriorly. Both the corneal epithelium and endothelium act as barriers to control the amount of fluid that is allowed to move into the tissue.
Superficial squamous cells of the epithelium are surrounded by a continuous band of tight junctions that prevent fluid from entering the stroma. Endothelial cells allow fluid to move between the cornea and the anterior chamber, but this movement is controlled by transport proteins in the cell membrane that regulate the osmotic gradient between the stroma and the aqueous.5
Signs

2. HSV disciform keratitis.
A host of slit-lamp findings may appear in response to corneal inflammatory events. Distinguishing among them can yield important clues to the cause and can help to guide treatment decisions.
• Superficial punctate keratitis (SPK). The most common sign of corneal inflammation, SPK can be caused by and associated with numerous ocular conditions, most commonly dry eye disease (figure 1). According to Ashley Behrens, M.D., and colleagues, superficial punctate keratitis is a common finding in patients who present with Level 2 dry eye disease.6 Additional signs of corneal inflammation include corneal swelling (epithelial or stromal edema), abnormal vessel growth (pannus and neovascularization), corneal infiltrates (accumulation of inflammatory WBCs), corneal ulceration and immune-mediated inflammation.
• Punctate epithelial erosions (PEE). A non-specific finding that appears clinically as tiny defects in the epithelium that stain with fluorescein, PEE are an early sign indicating epithelial compromise and are associated with many pathologic inflammatory conditions.
• Punctate epithelial keratitis (PEK). PEK appears as areas of focal, intraepithelial infiltrates, associated with areas of punctate staining.7
• Subepithelial infiltrates (SEI). SEIs generally occur after viral keratitis, but are also found in blepharitis and contact lens-related hypersensitivity. They result when chemical-signaling molecules draw white blood cells from the limbal vasculature into the avascular cornea.
• Corneal swelling. This may occur in different layers of the cornea; the type of swelling can be differentiated based on clinical appearance. Epithelial edema can present as epithelial microcysts, microcystic edema or epithelial bullae.
Epithelial microcysts are small, round, refractile lesions that originate in the basal layers of the epithelium and migrate toward the surface, where they stain with fluorescein.
Bullae form when excess fluid accumulates in the corneal epithelium, causing the surface epithelial layers to separate from the basement membrane. They appear as flat, pebble-like lesions and can present in a variety of configurations.3
Swelling of the corneal stroma manifests as an increase in stromal thickness. The appearance of edema in this region varies from a mild granular haze to a dense gray opacity and may produce folds in Descemet’s membrane if severe. Stromal edema may occur in response to compromised epithelial or endothelial cells.
• Neovascularization and pannus. Normally, the cornea is an avascular structure; however, neovascularization and pannus may occur in response to inflammatory disease.
Neovascularization can be caused by hypoxic conditions created by contact lens overwear, or it can form in response to inflammation from interstitial keratitis, viral keratitis or chemical burns.3
Pannus is a proliferation of fibrovascular tissue from the limbus that extends onto the cornea and is seen in conditions such as trachoma and superior limbic keratoconjunctivitis.3
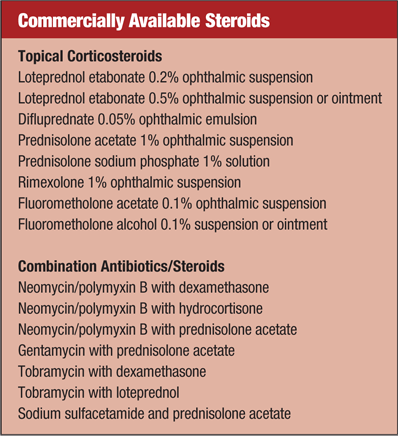 Common Causes
Common Causes
• Immune-mediated corneal inflammation. One common cause of corneal inflammation is an abnormal response from the host’s immune system. This can be the result of a hypersensitivity reaction to non-pathologic bacterial byproducts or by autoimmune disease directed at host tissue.8
Sterile keratitis is an inflammatory reaction to bacterial byproducts without direct corneal infection in contact lens wearers and lid margin disease. Bacteria colonize the contact lens surface producing toxins, which can be the catalyst for infiltrative keratitis. Hypersensitivity to contact lens solution can also occur.4 By a similar mechanism, colonization of the eyelids can cause phlyctenular keratoconjunctivitis or marginal keratitis.
Additionally, the host’s autoimmune system can be directed at the corneal tissue itself, as is believed to be the case in Mooren’s ulcer.3
• Infectious keratitis. Microbial keratitis is a major source of corneal inflammation and can be caused by bacterial, viral, fungal or parasitic invasion.
In response to bacterial infection, chemical-signaling molecules such as tumor necrosis factor-alpha and interleukin-1 cause neutrophils to escape the limbal blood vessels and move to the site of infection. Eventually macrophages arrive to phagocytize bacteria and degenerated neutrophils.3 The type of chemical mediators and immunologic cells involved depend on the source of the infection. Clinical signs of bacterial keratitis include corneal ulceration with stromal infiltration, corneal edema, anterior uveitis, hypopyon and perforation.7
The most common forms of viral keratitis are herpes simplex (HSV) and herpes zoster keratitis (HZO). HSV comes in several forms, including epithelial keratitis, neurotrophic keratitis, necrotizing stromal keratitis and endotheliitis:3
Epithelial keratitis is characterized by punctate or stellate epithelial lesions that progress to dendritic ulceration with characteristic terminal end-bulbs. The bed of the ulcers stain well with fluorescein and the margin stains with rose bengal. If not treated properly, lesions may progress to geographic ulceration.
Neurotrophic keratitis is characterized by a non-healing epithelial lesion with a gray, opaque stroma underneath, which may become thinned.
Necrotizing and non-necrotizing stromal keratitis presents with stromal necrosis, anterior uveitis and keratic precipitates.7 The inflammation present in HSV stromal keratitis is a hypersensitivity reaction to viral antigen (figure 2). Endotheliitis invariably displays keratic precipitates, stromal and epithelial edema, and iritis.3
As compared with HSV keratitis, herpes zoster keratitis includes epithelial keratitis—characterized by small, non-ulcerated, pseudodendritic lesions without terminal bulbs. Other presentations of viral keratitis include nummular keratitis, which presents with sub-epithelial infiltrates and stromal haze, disciform endotheliitis and anterior uveitis (figure 3).7
Fungal infections of the cornea typically have a gradual onset of pain and photophobia, and often occur in response to a traumatic injury. The cornea displays an infiltrative grayish/white ulcer and has less distinct, more feathery margins. Other associated findings include satellite lesions, a thick endothelial exudate, anterior uveitis and hypopyon.
Acanthamoeba keratitis is a parasitic infection of corneal tissue. Infiltration along corneal nerves, known as radial keratoneuritis, is pathognomonic for Acanthamoeba infection.7 The hallmark clinical presentation in the early stages of infection is advanced ocular pain out of proportion with signs. Epithelial pseudodendrites, anterior stromal infiltrates and a ring abscess may also be present.
As the Acanthamoeba infection progresses, subepithelial infiltrates can develop along corneal nerves with later formation of a ring stromal infiltrate. Patients who typically present with Acanthamoeba keratitis are typically contact lens wearers with poor hygiene and/or exposure to freshwater systems, pools and hot tubs.
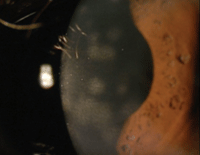
3. Nummular keratitis.
• Endothelial dysfunction. Any damage to the corneal endothelium can result in corneal edema, because these cells are responsible for regulating the osmotic gradient between the cornea and the anterior chamber. Causes of stromal swelling due to endothelial dysfunction include trauma during intraocular surgery, dystrophies of the corneal endothelium, iritis, viral keratitis or increased IOP. The most common of these is Fuchs’ dystrophy—focal accumulations of abnormal collagen on Descemet’s membrane (guttata) where the endothelial cells lose their ability to regulate ion movement.
Viral keratitis can cause direct inflammation of the endothelium, impacting its ability to regulate hydration.3
Swelling can also be seen in the presence of a healthy, intact corneal endothelium. With an intraocular pressure of 55mm Hg or greater due to angle closure glaucoma or viral infections (HSV, HZO), hydrostatic pressure from the aqueous becomes too great for the endothelial pumps to overcome and edema forms.3
Treatment
Treat corneal inflammation by identifying and targeting the specific underlying etiology. First, distinguish whether the corneal condition is infectious or inflammatory in nature. When in doubt, treat as infectious until proven otherwise. Once the infection is under control, anti-inflammatory drugs can always be added to reduce the risk of haze and scarring.
There are several classes of medications used in treating corneal inflammation, including corticosteroids (alone or in combination), cyclosporine A and hypertonic agents.
Steroids address inflammation in a variety of ways:
1. Steroids inhibit phospholipase A2, which prevents arachidonic acid from being released. Doing so inhibits the formation of prostaglandins, leukotrienes and lipoxins, which are powerful chemical mediators in the inflammatory cascade.9
2. Steroids inhibit pro-inflammatory cytokines, which call on other inflammatory cells to increase phagocytosis.
3. Steroids inhibit the production of complement molecules, which activate mast cells and basophils.
4. Steroids decrease the permeability of the capillary system and decrease fibroblast proliferation, thereby minimizing tissue damage.
There are many topical ocular steroids to choose from, differing in potency, bioavailability and safety. cyclosporine regulates inflammation by inhibiting the T-cell’s ability to produce pro-inflammatory signaling molecules. It is commonly used in dry eye syndrome and to prevent corneal graft rejection.
Hyperosmotic agents draw fluid out of the cornea by creating an osmotic gradient between the cornea and tear film. They are often used to improve comfort and vision in cases of bullous keratopathy; however, glycerin can also be used for diagnostic purposes.10 The most important consideration when treating corneal inflammation is to follow each patient until resolution. This ensures proper patient management and allows us the ability to monitor the condition and modify the treatment when necessary.
Corneal inflammation is a common finding and co-exists with other conditions of the ocular anatomy. Understanding the common symptoms and the individual clinical signs can better aid the practitioner in making the proper diagnosis.
In avascular structures such as the cornea, prompt treatment and continuous patient management can be key in providing an optically clear ocular surface.
Dr. McKenzie is a resident at the Louis Stokes Cleveland VA Medical Center. Dr. Whitley is the director of optometric services at Virginia Eye Consultants in Norfolk, Va.
1. Josephson JE, Zantos S, Caffery BE, et al. Differentiation of corneal complications observed in contact lens wearers. J Am Optom Assoc. 1988 Sep;59(9):679-85.
2. Catania L. Primary Care of the Anterior Segment. Connecticut: Appleton & Lange; 1995.
3. Krachmer JH, Mannis MJ, Holland EJ. Cornea. St. Louis, Mo.: Mosby/Elsevier; 2011.
4. Efron N. Sterile Keratitis. In: Contact Lens Complications. Oxford: Butterworth-Heinemann; 2004:168-70.
5. Levin LA, Adler FH. Adler’s physiology of the eye. Edinburgh: Saunders/Elsevier; 2011.
6. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006 Sep;25(8):900-7.
7. Kanski JJ. Clinical ophthalmology: A Systematic Approach. Edinburgh: Butterworth-Heinemann; 2011.
8. Garner A, Klintworth G. Pathobiology of Ocular Disease. England: Informa Healthcare; April 19, 1994.
9. Robbins SL, Kumar V. Basic Pathology. Philadelphia: Saunders; 2007.
10. Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. Oxford: Butterworth-Heinemann; 2008.


